The mainstay of therapy for liver cirrhosis remains the treatment of the underlying chronic liver disease, when possible, and prevention of superimposed hepatic insult, which could result in acute-on-chronic liver failure (ACLF). Early detection, control, and treatment of complications is essential; patients who develop complications should be referred for liver transplant evaluation.
Patients should be advised of the necessity for adequate nutrition, regular exercise, and the avoidance of hepatotoxins.[92]Perumpail BJ, Li AA, Cholankeril G, et al. Optimizing the nutritional support of adult patients in the setting of cirrhosis. Nutrients. 2017 Oct 13;9(10):E1114.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5691730
http://www.ncbi.nlm.nih.gov/pubmed/29027963?tool=bestpractice.com
Treatment of underlying chronic liver disease and prevention of superimposed hepatic insult
As cirrhosis is the pathological end-stage of any chronic liver disease, it is essential to treat the underlying causative condition in order to slow or halt the progression of cirrhosis. Oral direct-acting antivirals are considered a first-line treatment for chronic hepatitis C virus infection. One systematic review and meta-analysis found that early treatment with direct-acting antivirals reduced the recurrence of hepatocellular carcinoma, liver decompensation, and all-cause mortality, and prevented hepatocellular carcinoma in patients with compensated cirrhosis and without cirrhosis but did not prevent liver transplantation.[93]Yew KC, Tan QR, Lim PC, et al. Assessing the impact of direct-acting antivirals on hepatitis C complications: a systematic review and meta-analysis. Naunyn Schmiedebergs Arch Pharmacol. 2024 Mar;397(3):1421-31.
http://www.ncbi.nlm.nih.gov/pubmed/37728622?tool=bestpractice.com
Regimens depend on the genotype and presence or absence of cirrhosis.[94]American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Hepatitis C guidance 2023 update: recommendations for testing, managing, and treating hepatitis C virus infection. Oct 2023 [internet publication].
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciad319/7179952
Local guidance should be consulted. See Hepatitis C.
Antivirals may be indicated for patients with chronic hepatitis B.[84]European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 Aug;67(2):370-98.
https://www.journal-of-hepatology.eu/article/S0168-8278(17)30185-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28427875?tool=bestpractice.com
[95]Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018 Apr;67(4):1560-99.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5975958
http://www.ncbi.nlm.nih.gov/pubmed/29405329?tool=bestpractice.com
See Hepatitis B.
Superimposed hepatic insult may be prevented through the avoidance of alcohol and other hepatotoxic drugs (e.g., non-steroidal anti-inflammatory drugs [NSAIDs] and high doses of paracetamol [>2-3 g/day]), immunisation against hepatitis A and B for susceptible patients, management of metabolic risk factors, maintenance of adequate nutrition, and regular exercise.
Monitoring for complications
Cirrhosis is associated with serious complications including portal hypertension causing ascites (further complicated by spontaneous bacterial peritonitis and hepatic hydrothorax), gastro-oesophageal varices and portosystemic encephalopathy, acute kidney injury and hepatopulmonary syndromes, portopulmonary hypertension, ACLF, and hepatocellular carcinoma.
Prompt detection and treatment of these complications is essential in order to minimise related morbidity and mortality. Imaging tests include:
Abdominal ultrasound for the detection of ascites and surveillance for hepatocellular carcinoma
Upper gastrointestinal endoscopy for the detection of gastro-oesophageal varices
Abdominal ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) for the detection of hepatocellular carcinoma.
Other specialised tests may be required depending on individual symptoms.
Ascites
The most common complication of cirrhosis.
Every patient with new-onset ascites should undergo a diagnostic paracentesis: cell count with differential, albumin, and total protein should be measured in the ascitic fluid. Ascitic fluid should also be sent for culture and cytology.
The serum-ascites albumin gradient (SAAG) should be calculated: a SAAG of ≥11 g/L with low ascitic fluid total protein is consistent with portal hypertension secondary to cirrhosis.
Ascites develops in part due to activation of the renin-angiotensin-aldosterone system, resulting in sodium retention. Evidence suggests that severe dietary sodium restriction may be more harmful than beneficial, and many clinicians advise a no-added-salt diet rather than a low-salt diet.[101]Aithal GP, Palaniyappan N, China L, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021 Jan;70(1):9-29.
https://gut.bmj.com/content/70/1/9.long
http://www.ncbi.nlm.nih.gov/pubmed/33067334?tool=bestpractice.com
This means excluding additional table salt being added to foods and avoidance of foods with high salt content, such as crisps and some processed meals. This results in a moderate sodium restriction with daily intake of not more than 5 to 6.5 g.
First-line choice of diuretic should be spironolactone due to its effects on aldosterone and maintaining normal serum potassium.[101]Aithal GP, Palaniyappan N, China L, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021 Jan;70(1):9-29.
https://gut.bmj.com/content/70/1/9.long
http://www.ncbi.nlm.nih.gov/pubmed/33067334?tool=bestpractice.com
Furosemide may be added in patients who do not respond. Renal function and electrolytes should be monitored carefully when initiating diuretics and after dose escalation.
NSAIDs, ACE inhibitors, and other nephrotoxins should be avoided in patients with ascites.[90]Flamm SL, Wong F, Ahn J, et al. AGA clinical practice update on the evaluation and management of acute kidney injury in patients with cirrhosis: expert review. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2707-16.
https://www.cghjournal.org/article/S1542-3565(22)00829-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36075500?tool=bestpractice.com
Large volume refractory ascites
Some patients may develop large volume ascites refractory to medical treatment because of lack of efficacy, or unacceptable adverse effects or complications. These patients may require recurrent large-volume paracentesis (LVP) with albumin replacement for symptom control.[102]Bernardi M, Caraceni P, Navickis RJ, et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012 Apr;55(4):1172-81.
https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.24786
http://www.ncbi.nlm.nih.gov/pubmed/22095893?tool=bestpractice.com
Patients not suitable for liver transplantation should be considered for transjugular intrahepatic portosystemic shunt (TIPS) placement.[103]Benmassaoud A, Freeman SC, Roccarina D, et al. Treatment for ascites in adults with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013123.
https://www.doi.org/10.1002/14651858.CD013123.pub2
http://www.ncbi.nlm.nih.gov/pubmed/31978257?tool=bestpractice.com
Meta-analyses indicate that TIPS is more effective than paracentesis for control of refractory ascites, but is associated with a higher incidence of hepatic encephalopathy.[103]Benmassaoud A, Freeman SC, Roccarina D, et al. Treatment for ascites in adults with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013123.
https://www.doi.org/10.1002/14651858.CD013123.pub2
http://www.ncbi.nlm.nih.gov/pubmed/31978257?tool=bestpractice.com
[104]D'Amico G, Luca A, Morabito A, et al. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis. Gastroenterology. 2005 Oct;129(4):1282-93.
http://www.ncbi.nlm.nih.gov/pubmed/16230081?tool=bestpractice.com
[105]Saab S, Nieto JM, Lewis SK, et al. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004889.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004889.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/17054221?tool=bestpractice.com
[106]Albillos A, Bañares R, González M, et al. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005 Dec;43(6):990-6.
http://www.ncbi.nlm.nih.gov/pubmed/16139922?tool=bestpractice.com
[107]Salerno F, Cammà C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007 Sep;133(3):825-34.
https://www.doi.org/10.1053/j.gastro.2007.06.020
http://www.ncbi.nlm.nih.gov/pubmed/17678653?tool=bestpractice.com
[108]Chen RP, Zhu Ge XJ, Huang ZM, et al. Prophylactic use of transjugular intrahepatic portosystemic shunt aids in the treatment of refractory ascites: metaregression and trial sequential meta-analysis. J Clin Gastroenterol. 2014 Mar;48(3):290-9.
http://www.ncbi.nlm.nih.gov/pubmed/24030734?tool=bestpractice.com
[109]Bai M, Qi XS, Yang ZP, et al. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014 Mar 14;20(10):2704-14.
https://www.doi.org/10.3748/wjg.v20.i10.2704
http://www.ncbi.nlm.nih.gov/pubmed/24627607?tool=bestpractice.com
Overall mortality does not appear to differ between the two interventions, but TIPS may confer a modest benefit with respect to transplant-free survival.[103]Benmassaoud A, Freeman SC, Roccarina D, et al. Treatment for ascites in adults with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013123.
https://www.doi.org/10.1002/14651858.CD013123.pub2
http://www.ncbi.nlm.nih.gov/pubmed/31978257?tool=bestpractice.com
[104]D'Amico G, Luca A, Morabito A, et al. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis. Gastroenterology. 2005 Oct;129(4):1282-93.
http://www.ncbi.nlm.nih.gov/pubmed/16230081?tool=bestpractice.com
[105]Saab S, Nieto JM, Lewis SK, et al. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004889.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004889.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/17054221?tool=bestpractice.com
[106]Albillos A, Bañares R, González M, et al. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005 Dec;43(6):990-6.
http://www.ncbi.nlm.nih.gov/pubmed/16139922?tool=bestpractice.com
[107]Salerno F, Cammà C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007 Sep;133(3):825-34.
https://www.doi.org/10.1053/j.gastro.2007.06.020
http://www.ncbi.nlm.nih.gov/pubmed/17678653?tool=bestpractice.com
[108]Chen RP, Zhu Ge XJ, Huang ZM, et al. Prophylactic use of transjugular intrahepatic portosystemic shunt aids in the treatment of refractory ascites: metaregression and trial sequential meta-analysis. J Clin Gastroenterol. 2014 Mar;48(3):290-9.
http://www.ncbi.nlm.nih.gov/pubmed/24030734?tool=bestpractice.com
[109]Bai M, Qi XS, Yang ZP, et al. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014 Mar 14;20(10):2704-14.
https://www.doi.org/10.3748/wjg.v20.i10.2704
http://www.ncbi.nlm.nih.gov/pubmed/24627607?tool=bestpractice.com
TIPS placement can improve the nutritional status of patients with cirrhosis, indicated by an increase in ascites-free weight, body mass index, and muscle mass.[110]Gazda J, Di Cola S, Lapenna L, et al. The impact of transjugular intrahepatic portosystemic shunt on nutrition in liver cirrhosis patients: a systematic review. Nutrients. 2023 Mar 27;15(7):1617.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10096634
http://www.ncbi.nlm.nih.gov/pubmed/37049459?tool=bestpractice.com
One individual patient data meta-analysis found that TIPS treatment decreased the overall risk of a further decompensation and improved survival compared with standard of care.[111]Larrue H, D'Amico G, Olivas P, et al. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J Hepatol. 2023 Sep;79(3):692-703.
http://www.ncbi.nlm.nih.gov/pubmed/37141993?tool=bestpractice.com
One Cochrane systematic review and network meta-analysis reported that the overall certainty (quality) of evidence was very low, primarily because of unclear or high risk of bias in trials assessing interventions for the treatment of refractory ascites in patients with cirrhosis.[103]Benmassaoud A, Freeman SC, Roccarina D, et al. Treatment for ascites in adults with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013123.
https://www.doi.org/10.1002/14651858.CD013123.pub2
http://www.ncbi.nlm.nih.gov/pubmed/31978257?tool=bestpractice.com
[  ]
For adults with decompensated liver cirrhosis, how do treatments for ascites compare?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3068/fullShow me the answer Disease severity at the time of TIPS placement is an important predictive factor for outcomes.[112]Lebrec D, Giuily N, Hadengue A, et al. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French Group of Clinicians and a Group of Biologists. J Hepatol. 1996 Aug;25(2):135-44.
http://www.ncbi.nlm.nih.gov/pubmed/8878773?tool=bestpractice.com
]
For adults with decompensated liver cirrhosis, how do treatments for ascites compare?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3068/fullShow me the answer Disease severity at the time of TIPS placement is an important predictive factor for outcomes.[112]Lebrec D, Giuily N, Hadengue A, et al. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French Group of Clinicians and a Group of Biologists. J Hepatol. 1996 Aug;25(2):135-44.
http://www.ncbi.nlm.nih.gov/pubmed/8878773?tool=bestpractice.com
The prognosis of patients with refractory ascites is poor. If TIPS/transplantation are not viable options, then long-term drain placement for intermittent small-volume paracentesis may be considered as a palliative measure.[113]Macken L, Joshi D, Messenger J, et al. Palliative long-term abdominal drains in refractory ascites due to end-stage liver disease: a case series. Palliat Med. 2017 Jul;31(7):671-5.
http://www.ncbi.nlm.nih.gov/pubmed/27707955?tool=bestpractice.com
Prospective randomised controlled trials are warranted.
The use of vaptans (vasopressin V2-receptor antagonists) may have a slight beneficial effect on ascites and hyponatraemia, but they do not reduce mortality, liver complications, or renal failure.[114]Dahl E, Gluud LL, Kimer N, et al. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther. 2012 Oct;36(7):619-26.
http://www.ncbi.nlm.nih.gov/pubmed/22908905?tool=bestpractice.com
[115]Yan L, Xie F, Lu J, et al. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2015 Jun 9;15:65.
https://www.doi.org/10.1186/s12876-015-0297-z
http://www.ncbi.nlm.nih.gov/pubmed/26054761?tool=bestpractice.com
Gastro-oesophageal varices
Varices are present in 50% of patients with cirrhosis. Variceal bleeding is a life-threatening complication of cirrhosis with an associated mortality of 20% at 6 weeks.[116]Tripathi D, Stanley AJ, Hayes PC, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015 Nov;64(11):1680-704.
http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/25887380
http://www.ncbi.nlm.nih.gov/pubmed/25887380?tool=bestpractice.com
Patients with cirrhosis have historically been offered upper gastrointestinal endoscopy for screening of gastro-oesophageal varices at the time of diagnosis, and at 1- to 3-year intervals thereafter.[56]National Institute for Health and Care Excellence. Cirrhosis in over 16s: assessment and management. Sep 2023 [internet publication].
https://www.nice.org.uk/guidance/ng50
However, guideline recommendations vary regarding screening intervals.
The Baveno VII criteria suggest that screening endoscopy could be reserved for a subgroup of patients, based on liver stiffness measurement (LSM) and platelet count assessment.[54]de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII: renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
https://www.journal-of-hepatology.eu/article/S0168-8278(21)02299-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35120736?tool=bestpractice.com
In particular, patients with compensated cirrhosis, who have a liver stiffness <20 kPa (as measured by transient elastography) and platelet count >150 × 10⁹ cells/L, have a very low risk of having varices requiring treatment and can avoid screening endoscopy.[54]de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII: renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
https://www.journal-of-hepatology.eu/article/S0168-8278(21)02299-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35120736?tool=bestpractice.com
In practice, however, many centres still offer baseline endoscopy to all patients with liver cirrhosis.
In compensated patients with no or small varices at screening endoscopy, expanded Baveno VI criteria recommend screening for gastro-oesophageal varices at 1- to 3-year intervals thereafter.[87]de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015 Sep;63(3):743-52.
https://www.journal-of-hepatology.eu/article/S0168-8278(15)00349-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26047908?tool=bestpractice.com
Baveno VII consensus guidelines conclude that patients who avoid screening endoscopy can be followed up by yearly repetition of transient elastography and platelet count. If LSM increases (≥20 kPa) or platelet count declines (≤150 × 10⁹/L), these patients should undergo screening endoscopy.[54]de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII: renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-74.
https://www.journal-of-hepatology.eu/article/S0168-8278(21)02299-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35120736?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Oesophageal varices in a patient with portal hypertensionFrom the collection of Douglas G. Adler, MD [Citation ends].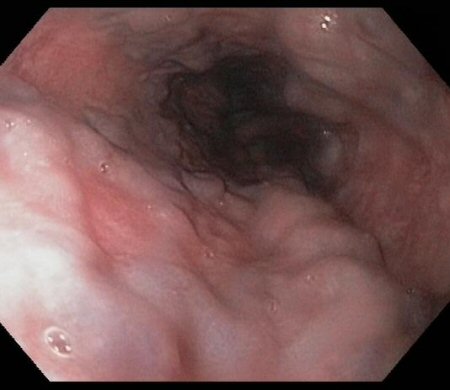
Primary prophylaxis of bleeding
Prophylaxis with either non-selective beta-blockers (propranolol, nadolol, or carvedilol) or endoscopic variceal ligation (EVL), which requires several sessions to obliterate varices, should be implemented if high-risk oesophageal varices are present, due to the increased risk of variceal bleeding.[57]Vadera S, Yong CWK, Gluud LL, et al. Band ligation versus no intervention for primary prevention of upper gastrointestinal bleeding in adults with cirrhosis and oesophageal varices. Cochrane Database Syst Rev. 2019 Jun 20;6:CD012673.
https://www.doi.org/10.1002/14651858.CD012673.pub2
http://www.ncbi.nlm.nih.gov/pubmed/31220333?tool=bestpractice.com
[  ]
How does band ligation compare with no intervention for adults with cirrhosis and esophageal varices in preventing upper gastrointestinal bleeding?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2700/fullShow me the answer High-risk varices include small varices with red signs, medium or large varices irrespective of Child-Pugh classification, or small varices in Child-Pugh C patients.[88]European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug;69(2):406-60.
https://www.journal-of-hepatology.eu/article/S0168-8278(18)31966-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29653741?tool=bestpractice.com
]
How does band ligation compare with no intervention for adults with cirrhosis and esophageal varices in preventing upper gastrointestinal bleeding?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2700/fullShow me the answer High-risk varices include small varices with red signs, medium or large varices irrespective of Child-Pugh classification, or small varices in Child-Pugh C patients.[88]European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug;69(2):406-60.
https://www.journal-of-hepatology.eu/article/S0168-8278(18)31966-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29653741?tool=bestpractice.com
Non-selective beta-blockers
Advantages of non-selective beta-blockers include low cost and ease of administration. Once a patient is on a non-selective beta-blocker, there is no need for repeat oesophagogastroduodenoscopy (OGD), and haemodynamic responders to non-selective beta-blockers have a lower incidence of decompensation and death. Disadvantages are that approximately 15% of patients may have absolute or relative contraindications to therapy, and another 15% require dose reduction or discontinuation attributed to common adverse effects (e.g., fatigue, weakness, and shortness of breath) that resolve upon discontinuation, but that may discourage patients and their physicians from using these drugs.
Monitoring haemodynamic response while treating a patient with beta-blockers is associated with a lower risk of variceal bleeding, but large cohort randomised controlled trials are required to confirm these findings.[117]Kerbert AJ, Chiang FW, van der Werf M, et al. Hemodynamic response to primary prophylactic therapy with nonselective beta-blockers is related to a reduction of first variceal bleeding risk in liver cirrhosis: a meta-analysis. Eur J Gastroenterol Hepatol. 2017 Apr;29(4):380-7.
http://www.ncbi.nlm.nih.gov/pubmed/28002118?tool=bestpractice.com
There is ongoing debate regarding the safety of non-selective beta-blockers in patients with cirrhosis and refractory ascites. The expanded Baveno VI criteria proposed that in patients with refractory ascites and (i) systolic blood pressure <90 mmHg, or (ii) serum creatinine >133 micromoles/L (>1.5 mg/dL), or (iii) hyponatraemia <130 millimoles/L, the beta-blocker should be reduced in dose or even temporarily discontinued.[87]de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015 Sep;63(3):743-52.
https://www.journal-of-hepatology.eu/article/S0168-8278(15)00349-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26047908?tool=bestpractice.com
However, there is evidence to suggest that the risk of harm may not be significant.[118]Bossen L, Krag A, Vilstrup H, et al. Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: Post Hoc analysis of three randomized controlled trials with 1198 patients. Hepatology. 2016 Jun;63(6):1968-76.
http://www.ncbi.nlm.nih.gov/pubmed/26599983?tool=bestpractice.com
[119]Sinha R, Lockman KA, Mallawaarachchi N, et al. Carvedilol use is associated with improved survival in patients with liver cirrhosis and ascites. J Hepatol. 2017 Jul;67(1):40-6.
http://www.ncbi.nlm.nih.gov/pubmed/28213164?tool=bestpractice.com
[120]Leithead JA, Rajoriya N, Tehami N, et al. Non-selective beta-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut. 2015 Jul;64(7):1111-9.
http://www.ncbi.nlm.nih.gov/pubmed/25281417?tool=bestpractice.com
[121]Zacharias AP, Jeyaraj R, Hobolth L, et al. Carvedilol versus traditional, non-selective beta-blockers for adults with cirrhosis and gastroesophageal varices. Cochrane Database Syst Rev. 2018 Oct 29;10:CD011510.
https://www.doi.org/10.1002/14651858.CD011510.pub2
http://www.ncbi.nlm.nih.gov/pubmed/30372514?tool=bestpractice.com
Endoscopic variceal ligation (EVL)
EVL is a local therapy that involves placing elastic bands around oesophageal varices until they are obliterated. EVL can theoretically be performed in the same session as screening endoscopy with hardly any contraindications. Disadvantages of EVL include the risks associated with sedation, plus the risk of causing dysphagia, oesophageal ulceration, strictures, and bleeding. Adverse effects associated with EVL may be severe, with reports of deaths resulting from EVL-induced bleeding ulcers. EVL is unable to prevent complications other than variceal haemorrhage. Surveillance endoscopies are necessary after variceal eradication, at least annually, to detect variceal recurrence.[122]Gralnek IM, Camus Duboc M, Garcia-Pagan JC, et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Nov;54(11):1094-120.
http://www.ncbi.nlm.nih.gov/pubmed/36174643?tool=bestpractice.com
Child-Pugh class C, ascites, or low prothrombin index are all indicators for high risk of early bleeding following EVL.[123]Garcia-Pagán JC, Bosch J. Endoscopic band ligation in the treatment of portal hypertension. Nat Clin Pract Gastroenterol Hepatol. 2005 Nov;2(11):526-35.
http://www.ncbi.nlm.nih.gov/pubmed/16355158?tool=bestpractice.com
The choice of whether to use non-selective beta-blockers or EVL should be based on local resources and expertise, patient preference and characteristics, contraindications, and adverse events.[87]de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015 Sep;63(3):743-52.
https://www.journal-of-hepatology.eu/article/S0168-8278(15)00349-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26047908?tool=bestpractice.com
One meta-analysis that included 1023 patients compared prophylactic EVL with beta-blockers and found that there was no difference between the treatments with regard to gastrointestinal haemorrhage, all-cause mortality, or haemorrhage-related mortality. While there was a decrease in variceal haemorrhage with EVL compared with beta-blockers (relative risk [RR] 0.72, 95% CI 0.4 to 0.96), variceal haemorrhage was not significantly different between the two groups when only high-quality trials were considered (RR 0.84, 95% CI 0.60 to 1.17).[124]Li L, Yu C, Li Y. Endoscopic band ligation versus pharmacological therapy for variceal bleeding in cirrhosis: a meta-analysis. Can J Gastroenterol. 2011 Mar;25(3):147-55.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3076033
http://www.ncbi.nlm.nih.gov/pubmed/21499579?tool=bestpractice.com
Subjective factors influence the physician's choice in selecting non-selective beta-blockers versus EVL, as illustrated in an interview-based study in which gastroenterologists who spent at least half their time performing endoscopy were more likely to choose EVL, whereas physicians who had a less procedural-based practice were more likely to choose non-selective beta-blockers.[125]Yan K, Bridges JF, Augustin S, et al. Factors impacting physicians' decisions to prevent variceal hemorrhage. BMC Gastroenterol. 2015 May 2;15:55.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4423490
http://www.ncbi.nlm.nih.gov/pubmed/25934271?tool=bestpractice.com
Transjugular intrahepatic portosystemic shunts (TIPS)
TIPS may be used as a secondary treatment option for oesophageal varices in cirrhosis but is not recommended for primary prophylaxis of variceal haemorrhage.[2]Kaplan DE, Ripoll C, Thiele M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024 May 1;79(5):1180-211.
https://journals.lww.com/hep/fulltext/2024/05000/aasld_practice_guidance_on_risk_stratification_and.22.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37870298?tool=bestpractice.com
[126]Jing L, Zhang Q, Chang Z, et al. Nonsurgical secondary prophylaxis of Eesophageal variceal bleeding in cirrhotic patients: a systematic review and network meta-analysis. J Clin Gastroenterol. 2021 Feb 1;55(2):159-68.
http://www.ncbi.nlm.nih.gov/pubmed/33122601?tool=bestpractice.com
Evidence obtained from trials of prophylactic surgical shunt therapy show a significantly higher rate of encephalopathy and a tendency for a higher mortality in patients randomised to shunt surgery.[127]D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995 Jul;22(1):332-54.
https://aasldpubs.onlinelibrary.wiley.com/doi/abs/10.1002/hep.1840220145
http://www.ncbi.nlm.nih.gov/pubmed/7601427?tool=bestpractice.com
[128]Li Y, Guo Y, Wang X, et al. Association between sarcopenia and hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: a systematic review and meta-analysis. Abdom Radiol (NY). 2024 Feb;49(2):575-85.
http://www.ncbi.nlm.nih.gov/pubmed/37980601?tool=bestpractice.com
Due to very low‐certainty evidence, conclusions cannot be drawn about the benefits and harms of portosystemic shunts compared with endoscopic interventions for people with cirrhosis and previous hypertensive portal bleeding (i.e., secondary prophylaxis).
[  ]
How do portosystemic shunts compare with endoscopic interventions for prevention of rebleeding in adults with cirrhosis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3462/fullShow me the answer
]
How do portosystemic shunts compare with endoscopic interventions for prevention of rebleeding in adults with cirrhosis?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3462/fullShow me the answer
Gastric varices
Gastric varices are present in about 20% of patients with cirrhosis and can present either as isolated gastric varices or as gastro-oesophageal varices.[129]Vine LJ, Subhani M, Acevedo JG. Update on management of gastric varices. World J Hepatol. 2019 Mar 27;11(3):250-60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6447419
http://www.ncbi.nlm.nih.gov/pubmed/30967903?tool=bestpractice.com
In the latter, the oesophageal varices extend below the cardia into the lesser curvature of the stomach (type 1 gastro-oesophageal varices [GOV 1]) or into the fundus (type 2 gastro-oesophageal varices [GOV 2]).[129]Vine LJ, Subhani M, Acevedo JG. Update on management of gastric varices. World J Hepatol. 2019 Mar 27;11(3):250-60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6447419
http://www.ncbi.nlm.nih.gov/pubmed/30967903?tool=bestpractice.com
They have different physiology and clinical characteristics compared with oesophageal varices. Gastric varices bleed less frequently but more significantly than oesophageal varices. Bleeding is less directly related to the degree of portal hypertension and more related to the size of the varix and wall tension.[129]Vine LJ, Subhani M, Acevedo JG. Update on management of gastric varices. World J Hepatol. 2019 Mar 27;11(3):250-60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6447419
http://www.ncbi.nlm.nih.gov/pubmed/30967903?tool=bestpractice.com
There is little literature regarding the management of gastric varices compared with oesophageal varices. Hence, most recommendations are based on expert opinion.[129]Vine LJ, Subhani M, Acevedo JG. Update on management of gastric varices. World J Hepatol. 2019 Mar 27;11(3):250-60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6447419
http://www.ncbi.nlm.nih.gov/pubmed/30967903?tool=bestpractice.com
Currently, non-selective beta-blockers are suggested for primary prevention of variceal bleeding from GOV 2 or isolated gastric varices. Treatment for GOV 1 should follow the above guidance for oesophageal varices.
Acute variceal haemorrhage
An episode of acute variceal haemorrhage should be managed as a medical emergency with intravascular volume support, blood transfusion (with the aim of keeping the haemoglobin around 70-80 g/L [7-8 g/dL]), and a combination of endoscopic and pharmacological therapy.[130]Odutayo A, Desborough MJ, Trivella M, et al. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: a systematic review and meta-analysis of randomised controlled trials. Lancet Gastroenterol Hepatol. 2017 May;2(5):354-60.
http://www.ncbi.nlm.nih.gov/pubmed/28397699?tool=bestpractice.com
[131]Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. 2019 Dec 3;171(11):805-22.
http://www.ncbi.nlm.nih.gov/pubmed/31634917?tool=bestpractice.com
Terlipressin (a vasopressin analogue), or somatostatin (or its analogue octreotide) should be initiated as soon as a variceal bleed is suspected and continued for 2-5 days if it is confirmed.[132]Garcia-Tsao G, Abraldes JG, Rich NE, et al. AGA clinical practice update on the use of vasoactive drugs and intravenous albumin in cirrhosis: expert review. Gastroenterology. 2024 Jan;166(1):202-10.
https://www.gastrojournal.org/article/S0016-5085(23)05143-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37978969?tool=bestpractice.com
Upper gastrointestinal endoscopy should be performed within 24 hours to confirm the diagnosis and allow treatment with EVL or sclerotherapy.[133]Lau JYW, Yu Y, Tang RSY, et al. Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med. 2020 Apr 2;382(14):1299-308.
https://www.doi.org/10.1056/NEJMoa1912484
http://www.ncbi.nlm.nih.gov/pubmed/32242355?tool=bestpractice.com
Short-term (up to 7 days) antibiotic prophylaxis should be instituted in all patients following a gastrointestinal haemorrhage (regardless of the presence of ascites) as this has been shown to decrease the rate of bacterial infections and increase survival.[134]Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, et al. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD002907.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002907.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/20824832?tool=bestpractice.com
[  ]
In people with cirrhosis and upper gastrointestinal bleeding, how does antibiotic prophylaxis affect outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.869/fullShow me the answer Ceftriaxone is the first choice in patients with decompensated cirrhosis, in those already on fluoroquinolone prophylaxis, and in hospital settings with high prevalence of fluoroquinolone-resistant bacterial infections. Oral fluoroquinolones should be used in the remaining patients.[135]Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006 Oct;131(4):1049-56.
https://www.gastrojournal.org/article/S0016-5085(06)01535-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17030175?tool=bestpractice.com
[136]Tandon P, Abraldes JG, Keough A, et al. Risk of bacterial infection in patients with cirrhosis and acute variceal hemorrhage, based on Child-Pugh class, and effects of antibiotics. Clin Gastroenterol Hepatol. 2015 Jun;13(6):1189-96.
https://www.cghjournal.org/article/S1542-3565(14)01710-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25460564?tool=bestpractice.com
]
In people with cirrhosis and upper gastrointestinal bleeding, how does antibiotic prophylaxis affect outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.869/fullShow me the answer Ceftriaxone is the first choice in patients with decompensated cirrhosis, in those already on fluoroquinolone prophylaxis, and in hospital settings with high prevalence of fluoroquinolone-resistant bacterial infections. Oral fluoroquinolones should be used in the remaining patients.[135]Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006 Oct;131(4):1049-56.
https://www.gastrojournal.org/article/S0016-5085(06)01535-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17030175?tool=bestpractice.com
[136]Tandon P, Abraldes JG, Keough A, et al. Risk of bacterial infection in patients with cirrhosis and acute variceal hemorrhage, based on Child-Pugh class, and effects of antibiotics. Clin Gastroenterol Hepatol. 2015 Jun;13(6):1189-96.
https://www.cghjournal.org/article/S1542-3565(14)01710-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25460564?tool=bestpractice.com
Life-threatening bleeding may be controlled with a Sengstaken-Blakemore tube or a Danis stent until haemostasis can be achieved endoscopically, or with TIPS, with or without embolisation.[137]Maiwall R, Jamwal KD, Bhardwaj A, et al. SX-Ella Stent Danis effectively controls refractory variceal bleed in patients with acute-on-chronic liver failure. Dig Dis Sci. 2018 Feb;63(2):493-501.
http://www.ncbi.nlm.nih.gov/pubmed/28780608?tool=bestpractice.com
Malnutrition and sarcopenia
It is recommended that patients hospitalised with cirrhosis receive formal dietician assessment, and steps should be taken to minimise the fasting period prior to procedures (e.g., by giving them a pre-bedtime snack or early-morning snack if the procedure will be in the late afternoon).[138]Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021 Sep;74(3):1611-44.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9134787
http://www.ncbi.nlm.nih.gov/pubmed/34233031?tool=bestpractice.com
Parenteral supplementation may be required for those who cannot meet their nutritional intake needs orally. Recommended protein intake for adults with cirrhosis is 1.2 to 1.5 g/kg (ideal body weight) per day, and 1.2 to 2 g/kg (ideal body weight) per day if critically ill.
Spontaneous bacterial peritonitis
A peritoneal fluid absolute neutrophil count >250 cells/mm³ is the accepted criterion for the diagnosis for spontaneous bacterial peritonitis.[88]European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug;69(2):406-60.
https://www.journal-of-hepatology.eu/article/S0168-8278(18)31966-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29653741?tool=bestpractice.com
Treatment is with intravenous antibiotics, such as cefotaxime or a fluoroquinolone, and intravenous human albumin solution. Albumin has been shown to reduce mortality in patients with cirrhosis with spontaneous bacterial peritonitis.[139]Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999 Aug 5;341(6):403-9.
http://www.nejm.org/doi/full/10.1056/NEJM199908053410603#t=article
http://www.ncbi.nlm.nih.gov/pubmed/10432325?tool=bestpractice.com
[140]Leache L, Gutiérrez-Valencia M, Saiz LC, et al. Meta-analysis: efficacy and safety of albumin in the prevention and treatment of complications in patients with cirrhosis. Aliment Pharmacol Ther. 2023 Mar;57(6):620-34.
http://www.ncbi.nlm.nih.gov/pubmed/36524316?tool=bestpractice.com
All patients who have survived an episode of spontaneous bacterial peritonitis require lifelong secondary antibiotic prophylaxis.
Patients with ascites and an ascitic fluid total protein level of ≤10 g/L are at high risk of developing spontaneous bacterial peritonitis, and should be considered for primary antibiotic prophylaxis.[88]European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug;69(2):406-60.
https://www.journal-of-hepatology.eu/article/S0168-8278(18)31966-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29653741?tool=bestpractice.com
[141]Loomba R, Wesley R, Bain A, et al. Role of fluoroquinolones in the primary prophylaxis of spontaneous bacterial peritonitis: meta-analysis. Clin Gastroenterol Hepatol. 2009 Apr;7(4):487-93.
http://www.ncbi.nlm.nih.gov/pubmed/19250986?tool=bestpractice.com
[142]Kamal F, Khan MA, Khan Z, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017 Oct;29(10):1109-17.
http://www.ncbi.nlm.nih.gov/pubmed/28763340?tool=bestpractice.com
There appears to be an increased risk of spontaneous bacterial peritonitis with the use of proton-pump inhibitors.[143]Hwang SJ, Lee DH, Koh SJ, et al. Correlation between proton pump inhibitors and the complications of liver cirrhosis: a systematic review and meta-analysis. Turk J Gastroenterol. 2022 Jan;33(1):44-52.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9128585
http://www.ncbi.nlm.nih.gov/pubmed/35040787?tool=bestpractice.com
High-quality evidence for this intervention is lacking and further trials are needed to establish whether antibiotic prophylaxis for people with cirrhosis is beneficial.[144]Iogna Prat L, Wilson P, Freeman SC, et al. Antibiotic treatment for spontaneous bacterial peritonitis in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2019 Sep 16;9:CD013120.
https://www.doi.org/10.1002/14651858.CD013120.pub2
http://www.ncbi.nlm.nih.gov/pubmed/31524949?tool=bestpractice.com
See Spontaneous bacterial peritonitis (Management approach).
Hepatic hydrothorax
Occurs in approximately 5% to 10% of patients with cirrhosis, usually in patients with ascites.[145]Badillo R, Rockey DC. Hepatic hydrothorax: clinical features, management, and outcomes in 77 patients and review of the literature. Medicine (Baltimore). 2014 May;93(3):135-42.
https://www.doi.org/10.1097/MD.0000000000000025
http://www.ncbi.nlm.nih.gov/pubmed/24797168?tool=bestpractice.com
Hepatic hydrothorax may cause dyspnoea. Management is similar to that of ascites. In some patients, long-term indwelling pleural catheters are required for symptomatic management.[146]Baig MA, Majeed MB, Attar BM, et al. Efficacy and safety of indwelling pleural catheters in management of hepatic hydrothorax: a systematic review of literature. Cureus. 2018 Aug 6;10(8):e3110.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6175258
http://www.ncbi.nlm.nih.gov/pubmed/30338185?tool=bestpractice.com
[147]Avula A, Acharya S, Anwar S, et al. Indwelling pleural catheter (IPC) for the management of hepatic hydrothorax: the known and the unknown. J Bronchology Interv Pulmonol. 2022 Jul 1;29(3):179-85.
http://www.ncbi.nlm.nih.gov/pubmed/34753862?tool=bestpractice.com
Patients with hepatic hydrothorax should be evaluated for liver transplantation.[148]Xiol X, Tremosa G, Castellote J, et al. Liver transplantation in patients with hepatic hydrothorax. Transpl Int. 2005 Jun;18(6):672-5.
https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1432-2277.2005.00116.x
http://www.ncbi.nlm.nih.gov/pubmed/15910292?tool=bestpractice.com
Portosystemic hepatic encephalopathy
Approximately 11% of patients with cirrhosis have hepatic encephalopathy at the time of cirrhosis diagnosis.[149]Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010 May;51(5):1675-82.
https://www.doi.org/10.1002/hep.23500
http://www.ncbi.nlm.nih.gov/pubmed/20186844?tool=bestpractice.com
Around 30% to 40% of patients with cirrhosis have an episode of hepatic encephalopathy during their illness, and there is a 30% to 40% chance of recurrence in the following year.[150]Sharma BC, Sharma P, Agrawal A, et al. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009 Sep;137(3):885-91, 891.e1.
https://www.doi.org/10.1053/j.gastro.2009.05.056
http://www.ncbi.nlm.nih.gov/pubmed/19501587?tool=bestpractice.com
Hepatic encephalopathy is characterised by changes in consciousness, behaviour, and personality with disorientation, drowsiness, forgetfulness, confusion, agitation, and eventual coma. Slurred speech, asterixis (liver flap), increased muscle tone, and extensor plantar reflexes may be present.
Precipitating factors include gastrointestinal haemorrhage, constipation, diarrhoea and vomiting, hypoglycaemia, and electrolyte imbalance; drugs (diuretics, sedatives) and medical procedures (paracentesis, TIPS); infection, anaemia, hypoxia, and hypotension. Gut-derived toxins such as ammonia and bacterial products that induce systemic inflammation cause hepatic encephalopathy.[3]Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023 May 9;329(18):1589-602.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10843851
http://www.ncbi.nlm.nih.gov/pubmed/37159031?tool=bestpractice.com
[18]Haj M, Rockey DC. Ammonia levels do not guide clinical management of patients with hepatic encephalopathy caused by cirrhosis. Am J Gastroenterol. 2020 May;115(5):723-8.
http://www.ncbi.nlm.nih.gov/pubmed/31658104?tool=bestpractice.com
There appears to be an increased risk of hepatic encephalopathy with the use of proton-pump inhibitors.[151]Shi D, Zhou Z, Dai Y, et al. Proton pump inhibitor therapy and hepatic encephalopathy risk in cirrhotic patients: a systematic review with meta-analysis. Clin Drug Investig. 2019 Sep;39(9):847-56.
http://www.ncbi.nlm.nih.gov/pubmed/31183628?tool=bestpractice.com
[152]Tantai XX, Yang LB, Wei ZC, et al. Association of proton pump inhibitors with risk of hepatic encephalopathy in advanced liver disease: a meta-analysis. World J Gastroenterol. 2019 Jun 7;25(21):2683-98.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6558434
http://www.ncbi.nlm.nih.gov/pubmed/31210719?tool=bestpractice.com
Treatment involves identification and correction of reversible precipitating factors and lactulose, used alone or in combination with antibiotics such as rifaximin.[153]Sharma BC, Sharma P, Lunia MK, et al. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013 Sep;108(9):1458-63.
http://www.ncbi.nlm.nih.gov/pubmed/23877348?tool=bestpractice.com
[154]Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016 May 6;(5):CD003044.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003044.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/27153247?tool=bestpractice.com
[155]Dhiman RK, Thumburu KK, Verma N, et al. Comparative efficacy of treatment options for minimal hepatic encephalopathy: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2020 Apr;18(4):800-12.e25.
https://www.cghjournal.org/article/S1542-3565(19)30969-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/31476436?tool=bestpractice.com
[156]Zacharias HD, Kamel F, Tan J, et al. Rifaximin for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2023 Jul 19;7(7):CD011585.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011585.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/37467180?tool=bestpractice.com
[157]Ahmed Z, Hassan M, Arif SF, et al. Comparative efficacy of treatment options for the prevention of post-TIPS hepatic encephalopathy: a systematic review and network meta-analysis. J Gastrointestin Liver Dis. 2023 Apr 1;32(1):70-6.
https://jgld.ro/jgld/index.php/jgld/article/view/4508
http://www.ncbi.nlm.nih.gov/pubmed/37004220?tool=bestpractice.com
[158]Nardelli S, Gioia S, Faccioli J, et al. Hepatic encephalopathy - recent advances in treatment and diagnosis. Expert Rev Gastroenterol Hepatol. 2023 Mar;17(3):225-35.
http://www.ncbi.nlm.nih.gov/pubmed/36843291?tool=bestpractice.com
[  ]
Can non-absorbable disaccharides help to prevent or treat hepatic encephalopathy in people with cirrhosis?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1475/fullShow me the answer[Evidence B]0fa90b9d-1299-47be-9f9f-c8d6ff20452eccaBCan non‐absorbable disaccharides help to prevent or treat hepatic encephalopathy in people with cirrhosis? Management of hepatic encephalopathy is not affected by ammonia levels.[3]Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023 May 9;329(18):1589-602.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10843851
http://www.ncbi.nlm.nih.gov/pubmed/37159031?tool=bestpractice.com
[18]Haj M, Rockey DC. Ammonia levels do not guide clinical management of patients with hepatic encephalopathy caused by cirrhosis. Am J Gastroenterol. 2020 May;115(5):723-8.
http://www.ncbi.nlm.nih.gov/pubmed/31658104?tool=bestpractice.com
Dietary protein restriction is not recommended.[159]Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014 Aug;60(2):715-35.
http://www.ncbi.nlm.nih.gov/pubmed/25042402?tool=bestpractice.com
]
Can non-absorbable disaccharides help to prevent or treat hepatic encephalopathy in people with cirrhosis?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1475/fullShow me the answer[Evidence B]0fa90b9d-1299-47be-9f9f-c8d6ff20452eccaBCan non‐absorbable disaccharides help to prevent or treat hepatic encephalopathy in people with cirrhosis? Management of hepatic encephalopathy is not affected by ammonia levels.[3]Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023 May 9;329(18):1589-602.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10843851
http://www.ncbi.nlm.nih.gov/pubmed/37159031?tool=bestpractice.com
[18]Haj M, Rockey DC. Ammonia levels do not guide clinical management of patients with hepatic encephalopathy caused by cirrhosis. Am J Gastroenterol. 2020 May;115(5):723-8.
http://www.ncbi.nlm.nih.gov/pubmed/31658104?tool=bestpractice.com
Dietary protein restriction is not recommended.[159]Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014 Aug;60(2):715-35.
http://www.ncbi.nlm.nih.gov/pubmed/25042402?tool=bestpractice.com
See Hepatic encephalopathy (Management approach).
Hepatorenal syndrome-acute kidney injury (HRS-AKI)
In patients with cirrhosis, the causes of acute kidney injury can be hypovolaemia, acute tubular necrosis, or hepatorenal syndrome with either acute kidney injury or acute kidney disease. Investigations to determine the cause include a careful history, physical examination, blood biochemistry, microscopic examination and chemical analysis of urine, select urine biomarkers and renal ultrasound.[90]Flamm SL, Wong F, Ahn J, et al. AGA clinical practice update on the evaluation and management of acute kidney injury in patients with cirrhosis: expert review. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2707-16.
https://www.cghjournal.org/article/S1542-3565(22)00829-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36075500?tool=bestpractice.com
HRS-AKI (formerly known as type-1 hepatorenal syndrome) can be diagnosed if patients meet the following criteria, as defined by the International Club of Ascites (ICA):[89]Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015 Apr;64(4):531-7.
https://www.doi.org/10.1136/gutjnl-2014-308874
http://www.ncbi.nlm.nih.gov/pubmed/25631669?tool=bestpractice.com
Cirrhosis with ascites
Diagnosis of AKI according to ICA-AKI criteria (i.e., increase in serum creatinine ≥26.4 micromoles/L [≥0.3 mg/dL] within 48 hours; or a percentage increase in serum creatinine ≥50% from baseline which is known, or presumed, to have occurred within the previous 7 days); the American Gastroenterological Association (AGA) also include a reduction of urine output to below 0.5 mL/kg/h for >6 hours[90]Flamm SL, Wong F, Ahn J, et al. AGA clinical practice update on the evaluation and management of acute kidney injury in patients with cirrhosis: expert review. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2707-16.
https://www.cghjournal.org/article/S1542-3565(22)00829-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36075500?tool=bestpractice.com
No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin
Absence of shock
No current or recent use of nephrotoxic drugs (NSAIDs, aminoglycosides, or iodinated contrast media)
No macroscopic signs of structural kidney injury. Structural kidney injury is indicated by proteinuria (>500 mg/day), microhaematuria (>50 red blood cells per high-power field), and/or abnormal renal ultrasonography.
European Association for the Study of the Liver (EASL) guidelines recommend using an adapted staging system for AKI that splits AKI stage 1 into stage 1A and 1B according to a serum creatinine value of <133 micromoles/L (<1.5 mg/dL) or ≥133 micromoles/L (≥1.5 mg/dL), respectively.[88]European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug;69(2):406-60.
https://www.journal-of-hepatology.eu/article/S0168-8278(18)31966-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29653741?tool=bestpractice.com
Measures can be taken to prevent HRS-AKI from developing in patients with cirrhosis, and include:[90]Flamm SL, Wong F, Ahn J, et al. AGA clinical practice update on the evaluation and management of acute kidney injury in patients with cirrhosis: expert review. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2707-16.
https://www.cghjournal.org/article/S1542-3565(22)00829-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36075500?tool=bestpractice.com
Advising patients to avoid alcohol
Monitoring serum creatinine levels and electrolytes when diuretics are given and avoiding excessive diuretics
Avoiding large-volume paracentesis without albumin replacement
Giving prophylactic antibiotics when infection is strongly suspected (investigations should include diagnostic paracentesis to evaluate for spontaneous bacterial peritonitis)
Avoiding use of non-selective beta-blockers and nephrotoxic drugs (e.g., ACE inhibitors, angiotensin-II receptor antagonists, and NSAIDs).
Once diagnosed, treatment of the precipitating cause should be initiated and any non-selective beta-blockers, diuretics, and NSAIDs held or stopped. Fluid losses should be replaced and fluid status monitored.[90]Flamm SL, Wong F, Ahn J, et al. AGA clinical practice update on the evaluation and management of acute kidney injury in patients with cirrhosis: expert review. Clin Gastroenterol Hepatol. 2022 Dec;20(12):2707-16.
https://www.cghjournal.org/article/S1542-3565(22)00829-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36075500?tool=bestpractice.com
Vasoconstrictors and albumin are recommended in all patients and should be expeditiously used. Terlipressin plus albumin should be considered as the first-line therapeutic option.[132]Garcia-Tsao G, Abraldes JG, Rich NE, et al. AGA clinical practice update on the use of vasoactive drugs and intravenous albumin in cirrhosis: expert review. Gastroenterology. 2024 Jan;166(1):202-10.
https://www.gastrojournal.org/article/S0016-5085(23)05143-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37978969?tool=bestpractice.com
Noradrenaline (norepinephrine) can be an alternative to terlipressin. However, there are several limitations associated with its use. Midodrine plus octreotide can be an option when terlipressin or noradrenaline are unavailable, but its efficacy is much lower than that of terlipressin.[88]European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug;69(2):406-60.
https://www.journal-of-hepatology.eu/article/S0168-8278(18)31966-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29653741?tool=bestpractice.com
[160]Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015 Aug;62(2):567-74.
https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.27709
http://www.ncbi.nlm.nih.gov/pubmed/25644760?tool=bestpractice.com
In one phase 3 trial to confirm the efficacy and safety of terlipressin plus albumin in adults with HRS-AKI, terlipressin was more effective than placebo in improving renal function, but was associated with serious adverse events, including respiratory failure, and cases of sepsis or septic shock.[161]Wong F, Pappas SC, Curry MP, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021 Mar 4;384(9):818-28.
https://www.nejm.org/doi/10.1056/NEJMoa2008290
http://www.ncbi.nlm.nih.gov/pubmed/33657294?tool=bestpractice.com
For further information, see Hepatorenal syndrome (Management approach).
Hyponatraemia
Patients with cirrhosis commonly develop hyponatraemia, especially with progression of liver disease. Hyponatraemia is usually well tolerated.
Initial management consists of discontinuation of diuretic therapy once serum sodium <130 mEq/L. Fluid restriction is usually necessary for serum sodium levels <115-120 mEq/L.
In patients with severe hyponatraemia treatment with vaptans may increase serum sodium, but their use does not reduce mortality, liver complications or renal failure.[88]European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018 Aug;69(2):406-60.
https://www.journal-of-hepatology.eu/article/S0168-8278(18)31966-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29653741?tool=bestpractice.com
[114]Dahl E, Gluud LL, Kimer N, et al. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther. 2012 Oct;36(7):619-26.
http://www.ncbi.nlm.nih.gov/pubmed/22908905?tool=bestpractice.com
Hyperkalaemia
Hyperkalaemia is frequently observed in patients with cirrhosis (12% to 14% of those hospitalised with cirrhosis).[44]Cai JJ, Wang K, Jiang HQ, et al. Characteristics, risk factors, and adverse outcomes of hyperkalemia in acute-on-chronic liver failure patients. Biomed Res Int. 2019 Feb 27;2019:6025726.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6415283
http://www.ncbi.nlm.nih.gov/pubmed/30937312?tool=bestpractice.com
It may indicate a poor prognosis and can present a challenge to treat.[44]Cai JJ, Wang K, Jiang HQ, et al. Characteristics, risk factors, and adverse outcomes of hyperkalemia in acute-on-chronic liver failure patients. Biomed Res Int. 2019 Feb 27;2019:6025726.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6415283
http://www.ncbi.nlm.nih.gov/pubmed/30937312?tool=bestpractice.com
[45]Wallerstedt S, Simrén M, Wahlin S, et al. Moderate hyperkalemia in hospitalized patients with cirrhotic ascites indicates a poor prognosis. Scand J Gastroenterol. 2013 Mar;48(3):358-65.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3581060
http://www.ncbi.nlm.nih.gov/pubmed/23298384?tool=bestpractice.com
The standard treatment of an insulin-glucose protocol to reduce serum potassium may be ineffective in the setting of cirrhosis, and therefore adjunct treatments may be preferred.[162]Lim AKH, Crnobrnja L, Metlapalli M, et al. Observational study of the relative efficacy of insulin-glucose treatment for hyperkalaemia in patients with liver cirrhosis. BMJ Open. 2021 Oct 22;11(10):e051201.
https://bmjopen.bmj.com/content/11/10/e051201.long
http://www.ncbi.nlm.nih.gov/pubmed/34686554?tool=bestpractice.com
Hepatopulmonary syndrome
Results from portal hypertension and occurs in at least 5% of patients awaiting liver transplantation.[163]Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome - a liver-induced lung vascular disorder. N Engl J Med. 2008 May 29;358(22):2378-87.
http://www.ncbi.nlm.nih.gov/pubmed/18509123?tool=bestpractice.com
Symptoms include dyspnoea and platypnoea. The diagnosis includes the finding of hypoxaemia with increased alveolar-arterial gradient, orthodeoxia, and the determination of intrapulmonary vascular dilation on contrast-enhanced echocardiography.
The presence of hepatopulmonary syndrome should prompt immediate evaluation for liver transplantation. The condition usually resolves following liver transplantation.[164]Grilo-Bensusan I, Pascasio-Acevedo JM. Hepatopulmonary syndrome: what we know and what we would like to know. World J Gastroenterol. 2016 Jul 7;22(25):5728-41.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4932208
http://www.ncbi.nlm.nih.gov/pubmed/27433086?tool=bestpractice.com
Portopulmonary hypertension
This complication is diagnosed when findings of unexplained pulmonary hypertension are present in association with portal hypertension. Unlike hepatopulmonary syndrome, this condition may not completely resolve following liver transplantation.
Patients with severe pulmonary hypertension that does not improve with the use of vasodilators such as epoprostenol, bosentan, iloprost, or sildenafil are not candidates for liver transplantation in view of the high risk of death associated with the procedure.
In one randomised controlled trial, macitentan, an endothelin-receptor antagonist, significantly reduced pulmonary vascular resistance in patients with portopulmonary hypertension compared with placebo.[165]Sitbon O, Bosch J, Cottreel E, et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med. 2019 Jul;7(7):594-604.
http://www.ncbi.nlm.nih.gov/pubmed/31178422?tool=bestpractice.com
Acute-on-chronic liver failure
ACLF is a severe form of acutely decompensated cirrhosis with organ failure and a high risk of short-term mortality.[166]European Association for the Study of the Liver. European Association for the Study of the Liver. EASL clinical practice guidelines on acute-on-chronic liver failure. J Hepatol. 2023 Aug;79(2):461-91.
https://www.journal-of-hepatology.eu/article/S0168-8278(23)00244-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37364789?tool=bestpractice.com
It is a heterogeneous syndrome; affected patients have features of hepatic failure (coagulopathy, elevated bilirubin) and may also have extrahepatic organ failure (kidney, lung, brain, or circulation). ACLF may be precipitated by alcohol use, viral hepatitis, drug-induced liver injury, surgery, ischaemia, or infection. It is a potentially reversible condition that may occur in people with chronic liver disease.
Treatment includes resuscitation, treatment of the precipitating factor, support of failing organs, and assessment for and early treatment of infection. Patients should be cared for in the intensive care unit. Some patients may benefit from early liver transplantation.[167]Bajaj JS, O'Leary JG, Lai JC, et al. Acute-on-chronic liver failure clinical guidelines. Am J Gastroenterol. 2022 Feb 1;117(2):225-52.
https://www.doi.org/10.14309/ajg.0000000000001595
http://www.ncbi.nlm.nih.gov/pubmed/35006099?tool=bestpractice.com
Treatment of shock in ACLF can be challenging. Society of Critical Care Medicine guidelines recommend using albumin as a resuscitation fluid over other fluids, particularly when serum albumin is below 3 mg/dL.[168]Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020 Mar;48(3):e173-91.
https://journals.lww.com/ccmjournal/Fulltext/2020/03000/Guidelines_for_the_Management_of_Adult_Acute_and.29.aspx
http://www.ncbi.nlm.nih.gov/pubmed/32058387?tool=bestpractice.com
Guidelines recommend aiming for a mean arterial pressure of 65 mmHg alongside invasive haemodynamic monitoring.[168]Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020 Mar;48(3):e173-91.
https://journals.lww.com/ccmjournal/Fulltext/2020/03000/Guidelines_for_the_Management_of_Adult_Acute_and.29.aspx
http://www.ncbi.nlm.nih.gov/pubmed/32058387?tool=bestpractice.com
[169]Karvellas CJ, Bajaj JS, Kamath PS, et al. AASLD practice guidance on acute-on-chronic liver failure and the management of critically ill patients with cirrhosis. Hepatology. 2024 Jun 1;79(6):1463-502.
https://journals.lww.com/hep/fulltext/2024/06000/aasld_practice_guidance_on_acute_on_chronic_liver.25.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37939273?tool=bestpractice.com
In patients with ACLF and hypotension, human albumin or crystalloids should be used for initial fluid therapy.[166]European Association for the Study of the Liver. European Association for the Study of the Liver. EASL clinical practice guidelines on acute-on-chronic liver failure. J Hepatol. 2023 Aug;79(2):461-91.
https://www.journal-of-hepatology.eu/article/S0168-8278(23)00244-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37364789?tool=bestpractice.com
If vasopressors are required, noradrenaline is more effective than dopamine in reversing hypotension. EASL recommends against the use of dopamine in patients with ACLF.[166]European Association for the Study of the Liver. European Association for the Study of the Liver. EASL clinical practice guidelines on acute-on-chronic liver failure. J Hepatol. 2023 Aug;79(2):461-91.
https://www.journal-of-hepatology.eu/article/S0168-8278(23)00244-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37364789?tool=bestpractice.com
Low-dose vasopressin can be added to noradrenaline in patients with ACLF who remain hypotensive despite fluid resuscitation. The American Association for the Study of Liver Diseases (AASLD) and EASL recommend noradrenaline as the first-line agent and vasopressin as the second-line agent for managing patients with hypotension.[166]European Association for the Study of the Liver. European Association for the Study of the Liver. EASL clinical practice guidelines on acute-on-chronic liver failure. J Hepatol. 2023 Aug;79(2):461-91.
https://www.journal-of-hepatology.eu/article/S0168-8278(23)00244-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37364789?tool=bestpractice.com
[169]Karvellas CJ, Bajaj JS, Kamath PS, et al. AASLD practice guidance on acute-on-chronic liver failure and the management of critically ill patients with cirrhosis. Hepatology. 2024 Jun 1;79(6):1463-502.
https://journals.lww.com/hep/fulltext/2024/06000/aasld_practice_guidance_on_acute_on_chronic_liver.25.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37939273?tool=bestpractice.com
The possible mortality benefit with the addition of vasopressin must be weighed against increased risk of digital ischaemia. As well as addressing the treatment of shock, guidelines also provide recommendations for managing endocrine, haematological, pulmonary, and renal features of ACLF in the intensive care unit setting.[168]Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020 Mar;48(3):e173-91.
https://journals.lww.com/ccmjournal/Fulltext/2020/03000/Guidelines_for_the_Management_of_Adult_Acute_and.29.aspx
http://www.ncbi.nlm.nih.gov/pubmed/32058387?tool=bestpractice.com
[169]Karvellas CJ, Bajaj JS, Kamath PS, et al. AASLD practice guidance on acute-on-chronic liver failure and the management of critically ill patients with cirrhosis. Hepatology. 2024 Jun 1;79(6):1463-502.
https://journals.lww.com/hep/fulltext/2024/06000/aasld_practice_guidance_on_acute_on_chronic_liver.25.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37939273?tool=bestpractice.com
Liver transplantation
Patients who develop complications of cirrhosis such as hepatocellular carcinoma or signs of decompensation (ascites, jaundice, variceal haemorrhage, portal systemic encephalopathy, hepatopulmonary syndrome, or hepatorenal syndrome) should be referred for liver transplant evaluation without delay. Transplant assessment may be a prolonged process and early referral is preferable. Some patients with ACLF may benefit from early liver transplantation.[167]Bajaj JS, O'Leary JG, Lai JC, et al. Acute-on-chronic liver failure clinical guidelines. Am J Gastroenterol. 2022 Feb 1;117(2):225-52.
https://www.doi.org/10.14309/ajg.0000000000001595
http://www.ncbi.nlm.nih.gov/pubmed/35006099?tool=bestpractice.com
Orthotopic liver transplantation remains the only curative treatment option for patients with decompensated cirrhosis.
Systems of liver allocation vary. In the US, liver allocation is based on the Model for End-Stage Liver Disease (MELD) score, which estimates the risk of 3-month mortality.[68]Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001 Feb;33(2):464-70.
http://www.ncbi.nlm.nih.gov/pubmed/11172350?tool=bestpractice.com
[
MELDNa scores (for liver transplantation listing purposes, not appropriate for patients under age 12 years) (SI units)
Opens in new window
]
A survival benefit from undergoing liver transplantation is seen when the MELD score is ≥15. In children aged <12 years, a complementary version of MELD score called Paediatric End-Stage Liver Disease (PELD) score is used.[170]Lucey MR, Furuya KN, Foley DP. Liver transplantation. N Engl J Med. 2023 Nov 16;389(20):1888-900.
http://www.ncbi.nlm.nih.gov/pubmed/37966287?tool=bestpractice.com
There is, however, a shortage of organ donors. In 2021, 9236 liver transplants were performed in the US, with the current waiting list standing at 11,185 candidates.[171]United Network for Organ Sharing. Transplant trends. 2022 [internet publication].
https://unos.org/data/transplant-trends
As a result, a significant number of patients die while waiting for an organ donor.
Presence of sarcopenia or frailty can affect liver transplantation outcomes. In one study, sarcopenia was found to be associated with adverse outcomes after liver transplantation in patients with decompensated cirrhosis.[172]Zhou D, Zhang D, Zeng C, et al. Impact of sarcopenia on the survival of patients undergoing liver transplantation for decompensated liver cirrhosis. J Cachexia Sarcopenia Muscle. 2023 Dec;14(6):2602-12.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10751414
http://www.ncbi.nlm.nih.gov/pubmed/37735907?tool=bestpractice.com
One systematic review of 34 studies concluded that patients with mild to moderate frailty or sarcopenia should be prioritised for liver transplantation due to increased mortality on the waiting list, but if the frailty or sarcopenia is severe, patients may be removed from the list as the prognosis is poor.[173]Ferreira AP, Machado MV. Impact of pretransplant frailty and sarcopenia on the post-transplant prognosis of patients with liver cirrhosis: a systematic review. Eur J Gastroenterol Hepatol. 2021 Dec 1;33(1s suppl 1):e883-97.
http://www.ncbi.nlm.nih.gov/pubmed/35048655?tool=bestpractice.com
Palliative care
As cirrhosis is a life-limiting condition, palliative care should be offered alongside other curative therapies, and may be relevant for patients with compensated cirrhosis and decompensated cirrhosis.[174]Tandon P, Walling A, Patton H, et al. AGA clinical practice update on palliative care management in cirrhosis: expert review. Clin Gastroenterol Hepatol. 2021 Apr;19(4):646-56.
https://www.cghjournal.org/article/S1542-3565(20)31561-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33221550?tool=bestpractice.com
The American Gastroenterological Association and the AASLD have both published guidelines on palliative care in patients with cirrhosis. These promote advanced care planning, assessment and management of symptoms (including pain, breathlessness, muscle cramps, sexual dysfunction, insomnia, daytime sleepiness, fatigue, pruritus, anxiety, and depression), screening for carer needs, and early liaison with local palliative care teams.[174]Tandon P, Walling A, Patton H, et al. AGA clinical practice update on palliative care management in cirrhosis: expert review. Clin Gastroenterol Hepatol. 2021 Apr;19(4):646-56.
https://www.cghjournal.org/article/S1542-3565(20)31561-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33221550?tool=bestpractice.com
[175]Rogal SS, Hansen L, Patel A, et al. AASLD practice guidance: palliative care and symptom-based management in decompensated cirrhosis. Hepatology. 2022 Sep;76(3):819-53.
https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.32378
http://www.ncbi.nlm.nih.gov/pubmed/35103995?tool=bestpractice.com
Particular note is given to chronic pain management in the setting of diminished liver function and novel treatments for refractory ascites.[175]Rogal SS, Hansen L, Patel A, et al. AASLD practice guidance: palliative care and symptom-based management in decompensated cirrhosis. Hepatology. 2022 Sep;76(3):819-53.
https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.32378
http://www.ncbi.nlm.nih.gov/pubmed/35103995?tool=bestpractice.com
 ]
Disease severity at the time of TIPS placement is an important predictive factor for outcomes.[112]
]
Disease severity at the time of TIPS placement is an important predictive factor for outcomes.[112]
 ]
High-risk varices include small varices with red signs, medium or large varices irrespective of Child-Pugh classification, or small varices in Child-Pugh C patients.[88]
]
High-risk varices include small varices with red signs, medium or large varices irrespective of Child-Pugh classification, or small varices in Child-Pugh C patients.[88] ]
]
 ]
Ceftriaxone is the first choice in patients with decompensated cirrhosis, in those already on fluoroquinolone prophylaxis, and in hospital settings with high prevalence of fluoroquinolone-resistant bacterial infections. Oral fluoroquinolones should be used in the remaining patients.[135][136]
]
Ceftriaxone is the first choice in patients with decompensated cirrhosis, in those already on fluoroquinolone prophylaxis, and in hospital settings with high prevalence of fluoroquinolone-resistant bacterial infections. Oral fluoroquinolones should be used in the remaining patients.[135][136] ]
[Evidence B] Management of hepatic encephalopathy is not affected by ammonia levels.[3][18] Dietary protein restriction is not recommended.[159]
]
[Evidence B] Management of hepatic encephalopathy is not affected by ammonia levels.[3][18] Dietary protein restriction is not recommended.[159]