Approach
Patients with cirrhosis often show no signs or symptoms for many years.[34] Around 40% of patients are diagnosed when they present with complications such as hepatic encephalopathy or ascites.[3] Screening for cirrhosis is recommended for patients with established chronic liver disease with abnormal liver enzymes, hepatic steatosis on imaging, or viral hepatitis, but not for the general population.[3]
Cirrhosis can be identified by presence of clinical findings plus laboratory tests that reflect the underlying pathophysiology.[35] Simple laboratory tests and clinical findings that increase the likelihood of cirrhosis have been discussed in one meta-analysis.[35] These include: presence of ascites, platelet count of <160 × 10⁹/L (<160,000/microlitre), spider naevi, or a combination of simple laboratory tests with the Bonacini cirrhosis discriminant score of >7.[35]
The evaluation of a patient with suspected chronic liver disease and cirrhosis should begin with a detailed history identifying the presence of risk factors for the different causes of cirrhosis. Patients should then undergo a thorough physical examination in order to elicit any signs of chronic liver disease or complications of cirrhosis.
A full panel of blood tests should be undertaken to establish the aetiology of chronic liver disease and to ascertain the degree of disease severity. Liver imaging and screening endoscopy may be required. Liver biopsy, which was performed as a standard test, is now being increasingly replaced with non-invasive tests.[3]
Cirrhosis should be differentiated from non-cirrhotic conditions that can also lead to portal hypertension. These include disorders such as constrictive pericarditis; vascular disorders such as Budd-Chiari syndrome, portal vein and splenic vein thrombosis, and inferior vena cava obstruction; infectious agents such as schistosomiasis; and sarcoidosis, nodular regenerative hyperplasia, and idiopathic portal hypertension, also known as hepatoportal sclerosis. It is important to exclude exposure to substances that may cause portal hypertension such as vitamin A intoxication, arsenic, and vinyl chloride toxicity.
Certain non-hepatic conditions may lead to the development of cirrhosis, where congestive heart failure or cardiopulmonary disease lead to passive hepatic congestion, which may lead to cirrhosis with time.
History
Presenting features[35]
Patients with cirrhosis may be asymptomatic or have non-specific constitutional symptoms, such as fatigue, weakness, and weight loss, as well as recurrent infections and decreased libido.
Symptoms of decompensation include:
Abdominal distension due to ascites
Vomiting of blood (haematemesis) and black stool (melaena) secondary to variceal haemorrhage
Altered mental status in hepatic encephalopathy
Peripheral oedema
Jaundice.
Less common symptoms associated with pulmonary complications of portal hypertension include dyspnoea on exertion. In patients with hepatopulmonary syndrome, platypnoea (shortness of breath with sitting up) and orthodeoxia (deoxygenation with sitting up) are classically described; patients may also develop clubbing and cyanosis. In portopulmonary hypertension, patients may develop syncope and chest pain/pressure.
Past medical history
Check whether the patient has already been diagnosed with a chronic liver disease.
Elicit any history of metabolic syndromes (diabetes, dyslipidaemia, obesity, hypertension) or autoimmune disorders.
Knowledge of the patient's past medical history may be helpful in identifying exposure to hepatotoxic drugs.
Patients should be asked about previous history of blood transfusion.
Drug history
A complete drug history should be taken. Long-term use of certain drugs such as methotrexate and amiodarone has been implicated in the development of liver cirrhosis.[36] However, one evidence-based review found that advanced liver fibrosis and cirrhosis previously attributed to methotrexate were caused by metabolic liver disease or other chronic liver diseases and not by methotrexate itself.[37]
It is important to elicit the use of over-the-counter drugs, vitamins, and herbal and dietary supplementation, which may account for liver injury and may not be volunteered by the patient.[38]
Family history
A family history of haemochromatosis, Wilson's disease, or alpha-1 antitrypsin deficiency provides an important diagnostic clue.
Chronic hepatitis B may be transmitted from mother to child, and asking about a family history of viral hepatitis is important.[30]
Patients should be asked whether there is a family history of liver cirrhosis or hepatocellular carcinoma as genetic susceptibility to liver injury is likely to play a role in risk.[39]
A history of metabolic risk factors in the family, particularly diabetes, should raise suspicion for metabolic dysfunction-associated steatotic liver disease.
Social history and risk factors
Patients should be asked sensitively about risk-taking behaviours, such as intravenous drug use, unprotected intercourse, and tattoos.[33]
A detailed alcohol history should be taken in order to assess the patient's level and pattern of alcohol consumption, and the number of units consumed per week should be documented.[29]
A thorough travel history should also be taken, including country of birth and ethnic origin of parents, as well as a history of dental or surgical procedures performed abroad.[32]
A history of patterns of weight gain/loss should be taken.
Physical examination
Chronic liver disease and cirrhosis have a variety of physical characteristics, some of which are specific to the underlying causative disease.
Hand and nail features
Leukonychia (white nails) secondary to hypoalbuminaemia
Polished nails secondary to excessive scratching in pruritus
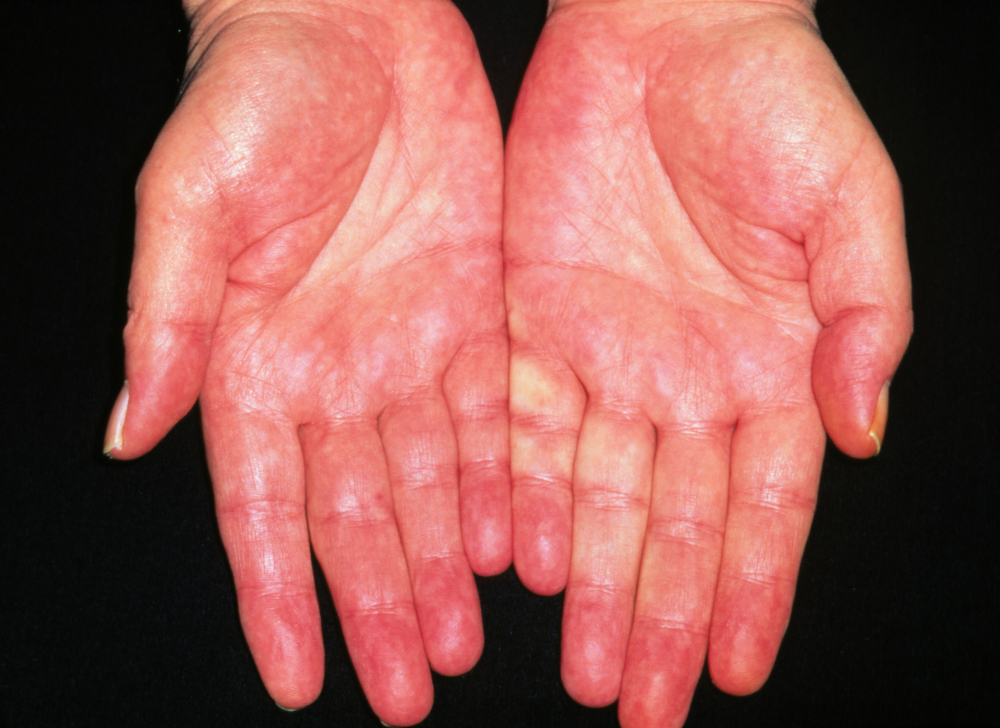
Palmar erythema
Spider naevi
Bruising
Finger clubbing and cholesterol deposits in palmar creases in primary biliary cholangitis
Dupuytren contracture in alcohol-related liver disease
Cyanosis and finger clubbing in patients with hepatopulmonary syndrome.[35][40]
[Figure caption and citation for the preceding image starts]: Liver palms erythema of adult alcoholicDr P. Marazzi / Science Photo Library; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Preoperative view of a small finger flexion contractureFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Preoperative view of a small finger flexion contractureFrom the collection of Dr C.M. Rodner; used with permission [Citation ends].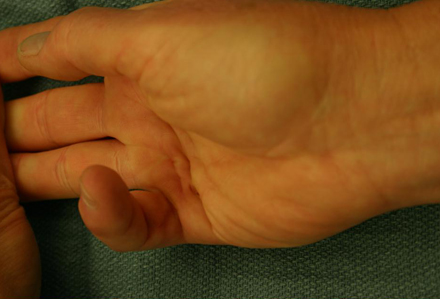
Facial features
Telangiectasia
Spider naevi
Bruising
Rhinophyma (lobulated and hypertrophied appearance of the nose secondary to sebaceous gland hyperplasia)
Parotid gland swelling
Glossitis
Skin has the appearance of a US dollar note ('paper-money' skin, randomly distributed thready blood vessels)
Seborrhoeic dermatitis
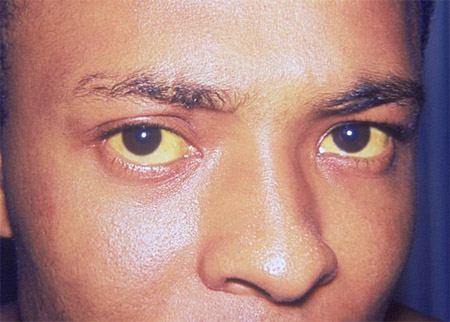
Jaundiced sclerae
Xanthelasma in primary biliary cholangitis.[Figure caption and citation for the preceding image starts]: Icterus or jaundiceCDC. Dr Thomas F. Sellers/Emory University; used with permission [Citation ends].
Chest wall features
Gynaecomastia (tender and firm enlarged breast bud) and loss of secondary sexual hair in men
Breast atrophy in women.
Abdominal features
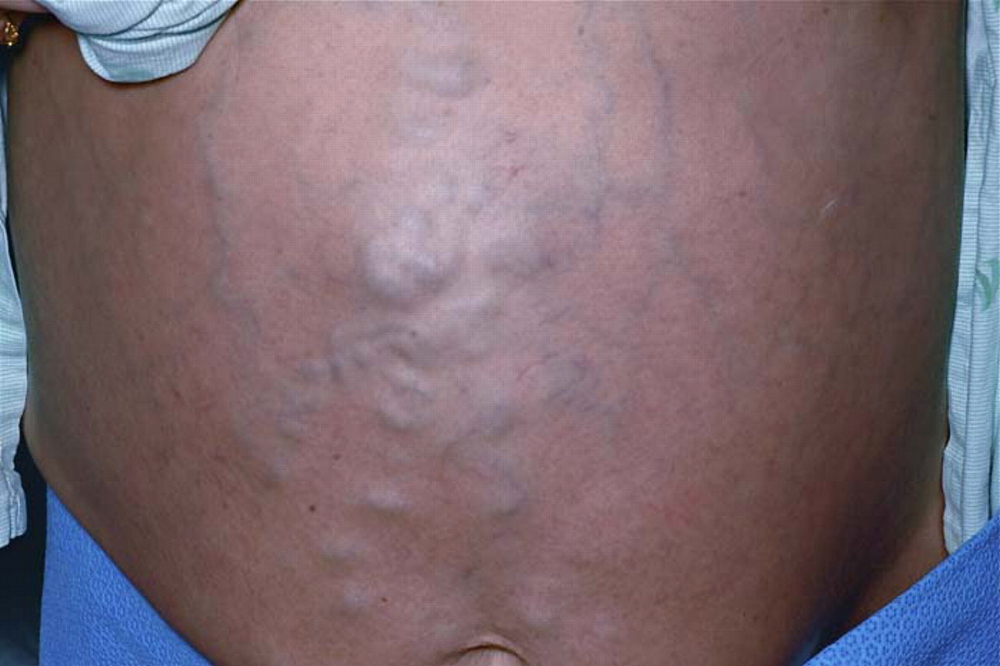
Collateral circulation of the abdominal wall around the umbilicus (caput medusa)
Bruising
Hepatomegaly
Splenomegaly
Abdominal distension (particularly in the flanks) with shifting dullness and fluid thrill secondary to ascites
Hepatic bruit may be present with a vascular hepatoma
Loss of secondary sexual hair and testicular atrophy in men.
[Figure caption and citation for the preceding image starts]: Caput medusa: dilated superficial (superior and inferior) epigastric veins radiating from a central large venous varixSingh NK, Cheema U, Khalil A. Caput medusae. Case Reports. 2010;2010:bcr0320102795; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Ascites. View of the abdomen of a female patient with alcohol-related liver disease and cirrhosis, showing swelling due to ascites (accumulation of fluid in the peritoneal cavity), jaundice (yellowing of the skin), and bruisingScience Photo Library; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Ascites. View of the abdomen of a female patient with alcohol-related liver disease and cirrhosis, showing swelling due to ascites (accumulation of fluid in the peritoneal cavity), jaundice (yellowing of the skin), and bruisingScience Photo Library; used with permission [Citation ends].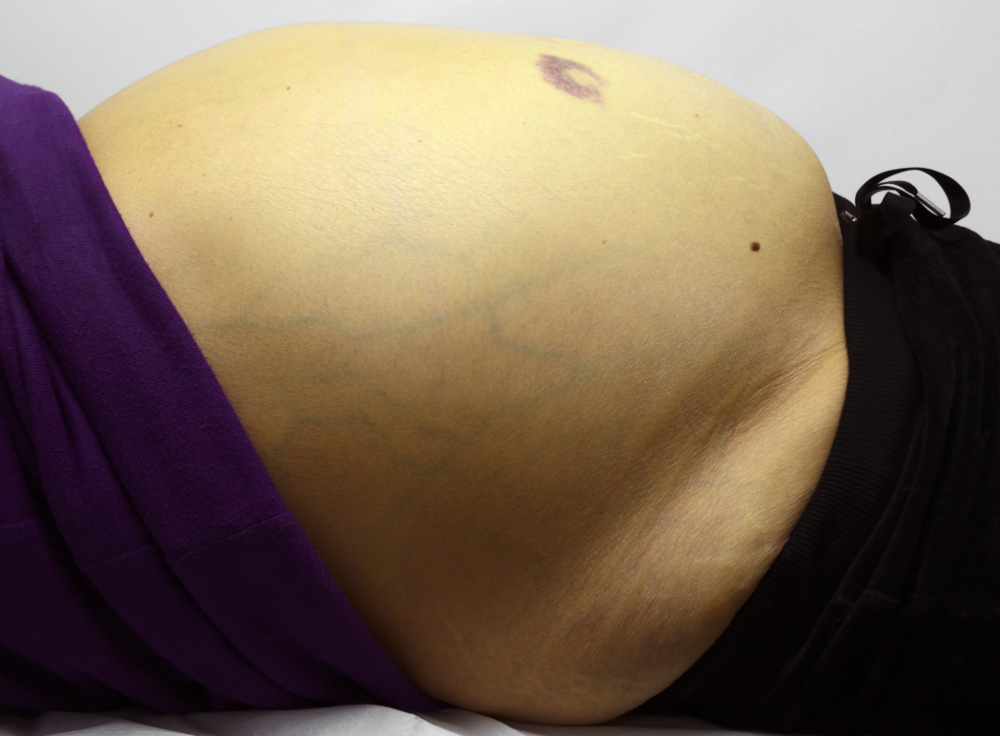
Other physical findings include hepatic fetor, muscle wasting, and peripheral oedema, and findings of elevated right heart pressure such as elevated jugular venous pulse, increased split of the second heart sound, and pulsatile liver in patients with portopulmonary hypertension.
Blood tests
All patients should receive a liver screen on presentation in order to identify the underlying cause and severity of the cirrhosis.
Liver function tests
These tests show characteristic results depending on the nature of the hepatic insult.
Aminotransferases (aspartate aminotransferase [AST], alanine aminotransferase [ALT]) levels increase with hepatocellular damage and are usually elevated to some degree.
Normal AST and ALT levels do not preclude the diagnosis of cirrhosis.[41]
Aminotransferase levels bear little to no relationship to frequency of complications or death.[42]
ALT levels are greater than those of AST in most chronic liver diseases (except for alcohol-related liver disease), but this finding may be reversed with progression of liver disease.
An AST/ALT ratio of ≥1 is thought to be a predictor of cirrhosis.[43]
Alkaline phosphatase and gamma-glutamyl transferase (GGT) levels increase in cholestasis (resulting from primary biliary cholangitis and primary sclerosing cholangitis), with minimal derangement of AST and ALT.
Total bilirubin may be normal in patients with compensated cirrhosis, but as the cirrhosis progresses, serum levels generally rise.
It is important to recognise that these tests can be elevated in conditions other than liver disease. For example, aminotransferases can be elevated in systemic diseases without primary liver involvement, such as thyroid disease, muscle disorders (including cardiac ischaemia), and coeliac disease. Alkaline phosphatase can be elevated with bone disease and total bilirubin will be elevated in the setting of haemolysis.
Gamma-glutamyl transferase (GGT)
Increase in this liver microsomal enzyme represents enzyme activation, which can be induced by alcohol and certain drugs, and is also observed in metabolic dysfunction-associated steatotic liver disease (MASLD).
GGT is increased in cholestasis along with alkaline phosphatase (ALP).
GGT is not significantly present in bone, such that concomitant elevated GGT and ALP indicate the liver as the source of the ALP.
Albumin
A decrease in serum albumin is a marker of hepatic synthetic dysfunction.
Electrolytes
Hyponatraemia is a common finding in patients with cirrhosis with associated ascites, and worsens as the liver disease progresses.
Hyperkalaemia is frequently observed in patients with cirrhosis (12% to 14% of those hospitalised with cirrhosis).[44] It may indicate a poor prognosis, and can present a challenge to treat.[44][45]
Full blood count and clotting
Prolongation of the prothrombin time is a marker of hepatic synthetic dysfunction. (Although prothrombin time can be prolonged in patients with vitamin K deficiency, it is readily reversed by vitamin K replacement, which does not occur in the setting of hepatic dysfunction.)
Coagulopathy in liver disease is complex. A prolonged prothrombin time does not necessarily mean that the patient is at increased risk of bleeding, and correction of coagulopathy with blood products outside the context of acute bleeding is not usually warranted.[46] Expert consensus recommends that International Normalised Ratio testing is not required for diagnostic or therapeutic paracentesis or diagnostic endoscopy, and that plasma transfusion should be avoided for all paracenteses and gastroscopy.[47]
The presence of thrombocytopenia (platelet count <150,000/microlitre) is the most sensitive and specific laboratory finding for the diagnosis of cirrhosis in the setting of chronic liver disease and results from portal hypertension with hypersplenism and platelet sequestration.[48]
Viral serology
Presence of immunoglobulin G (IgG) antibodies to hepatitis C virus, confirmed with hepatitis C virus RNA, is indicative of chronic hepatitis C infection.[49] Hepatitis C genotype is ordered once infection is confirmed in order to guide treatment decisions.
A detectable hepatitis B surface antigen (HBsAg) or viraemia on a highly sensitive hepatitis B DNA assay indicates chronic hepatitis B infection.[49] Hepatitis B e-antigen/e-antibody, genotype, and viral load should be measured to assess disease phase and guide further treatment.[49]
Iron studies
The initial screening tests for haemochromatosis are total iron and TIBC, in order to calculate the transferrin saturation (iron/TIBC), and serum ferritin. If the transferrin saturation and ferritin are elevated, HFE genotyping is performed.[50]
Auto-antibody screen
Auto-antibodies: antinuclear (ANA), antismooth muscle (SMA), and liver kidney microsomal antibody (anti-LKM) for autoimmune hepatitis; antimitochondrial (AMA) for primary biliary cholangitis, more specifically the M2 antibody.
Serum immunoglobulins
Assessment of serum immunoglobulins (IgA, IgG, and IgM) should be undertaken. Levels are frequently elevated in patients with cirrhosis.[51]
Ceruloplasmin
Serum ceruloplasmin is decreased in Wilson's disease.
Alpha-1 antitrypsin
Approximately 15% of adults with alpha-1 antitrypsin deficiency develop cirrhosis.[52]
Plasma alpha-1 antitrypsin levels are used as a screening test.
Alpha-1 antitrypsin is an acute phase protein and may be elevated in inflammation.
Further testing can be done to confirm the diagnosis with protein electrophoresis and phenotyping.
Alpha-fetoprotein
The finding of elevated alpha-fetoprotein in a patient with cirrhosis should raise concern for the development of hepatocellular carcinoma.[53] However, this tumour marker may also be elevated on the basis of chronic liver disease and inflammation in the absence of hepatocellular carcinoma; therefore, cross-sectional imaging is indicated to exclude the presence of hepatic lesions.
Endoscopy
An upper gastrointestinal endoscopy should be performed in selected patients with cirrhosis to screen for oesophago-gastric varices. The selection of these patients is based on non-invasive assessment of their liver comprising platelet count and liver stiffness measurement (LSM).[54] In particular, patients with compensated cirrhosis, who have a liver stiffness <20 kPa (as measured by transient elastography) and platelet count >150 × 10⁹ cells/L, have a very low risk of having varices requiring treatment and can avoid screening endoscopy.[54] In practice, however, many centres offer baseline endoscopy to all patients with liver cirrhosis. Meta-analysis has shown that ultra-thin gastroscopy may be a better tolerated method for screening for varices where indicated.[55]
Patients with cirrhosis have historically been offered baseline upper gastrointestinal endoscopy for screening of gastro-oesophageal varices at the time of diagnosis, and at 1- to 3-year intervals thereafter.[56] However, guideline recommendations vary regarding screening intervals.
Primary prophylaxis (to prevent variceal bleeding) with either non-selective beta-blockers (propranolol, nadolol, or carvedilol) or endoscopic variceal ligation (EVL), which requires several sessions to obliterate varices, should be implemented if high-risk gastro-oesophageal varices are present.[57]
[  ]
One meta-analysis reported that, for primary prevention of variceal bleeding, variceal-band ligation plus beta-blocker resulted in a lower bleed rate compared with beta-blocker alone, but was also associated with a higher adverse event rate.[58] One competing-risk meta-analysis reported no added benefit of EVL plus non-selective beta-blocker combination over non-selective beta-blocker alone in patients with compensated cirrhosis and high-risk varices.[59] A significant improvement in survival was observed with non-selective beta-blocker alone, thus making it a preferred option for preventative therapy.[59] Combination therapy is not recommended for primary prophylaxis.
]
One meta-analysis reported that, for primary prevention of variceal bleeding, variceal-band ligation plus beta-blocker resulted in a lower bleed rate compared with beta-blocker alone, but was also associated with a higher adverse event rate.[58] One competing-risk meta-analysis reported no added benefit of EVL plus non-selective beta-blocker combination over non-selective beta-blocker alone in patients with compensated cirrhosis and high-risk varices.[59] A significant improvement in survival was observed with non-selective beta-blocker alone, thus making it a preferred option for preventative therapy.[59] Combination therapy is not recommended for primary prophylaxis.
Imaging
Signs of cirrhosis or portal hypertension may be detected using ultrasound, computed tomography (CT) scan, and magnetic resonance imaging (MRI).[60][61] The choice of imaging modality is dependent on the pathology requiring investigation and physician preference.
Ultrasound with Doppler assessment of flow within the hepatic vasculature is the preferred test for the initial evaluation of patients with suspected cirrhosis. It avoids radiation and contrast risks associated with other imaging modalities.
If there are features suspicious for hepatocellular carcinoma on ultrasound, or if the patient has unexplained abdominal pain, further evaluation with CT or MRI is recommended.[62]
Liver surface nodularity or a small liver with or without hypertrophy of the left/caudate lobe is detectable on ultrasound, CT, and MRI. Signs of advanced cirrhosis may be detected using ultrasound, CT scan, or MRI.
There is no radiological test sensitive enough to be used as the sole diagnostic tool for cirrhosis. However, the radiological findings described above, in combination with a strong clinical suspicion, suffice for the diagnosis of cirrhosis without the need for a confirmatory liver biopsy.
Imaging studies in patients with cirrhosis are an important tool for early detection of hepatocellular carcinoma and are routinely used for surveillance of this condition.
Liver biopsy
Liver biopsy remains the most specific and sensitive test for the diagnosis of cirrhosis. However, it is not necessary in patients with advanced liver disease and typical clinical, laboratory, and/or radiological findings of cirrhosis, unless there is a need to determine the degree of inflammation.
In addition to confirming the diagnosis, liver biopsy may help to determine the aetiology of the underlying liver disease, although this is not always possible as characteristic features of the primary insult (e.g., MASLD or autoimmune hepatitis) may no longer be detectable by the time the procedure is carried out.
Liver biopsy is also helpful in diagnosing coexistent liver diseases (e.g., steatotic liver disease and viral hepatitis, haemochromatosis and viral hepatitis) and autoimmune overlap syndromes, as well as infiltrative and infectious disorders.
Liver biopsy may help guide management of specific causes of chronic liver disease and cirrhosis. In patients with cirrhosis and hepatic lesions, liver biopsy may be necessary in some cases in order to differentiate benign lesions from primary liver cancer and metastatic liver disease.
Liver biopsy is associated with risk of bleeding, perforation, and pneumothorax, among other complications.[63]
Severity scoring
The two most commonly used scoring systems to determine disease severity are the Child-Pugh-Turcotte (CPT) and, more recently, the Model of End-Stage Liver Disease (MELD). Other scoring systems continue to be evaluated.[64][65][66][67]
Child-Pugh-Turcotte (CPT)
Based on the presence of ascites and hepatic encephalopathy, serum bilirubin, albumin, and clotting (prothrombin time and international normalised ratio [INR]) and is divided into Child A, B, and C with increasing disease severity. [Figure caption and citation for the preceding image starts]: Child-Pugh-Turcotte scoring systemFrom the collection of Dr Keith Lindor; used with permission [Citation ends].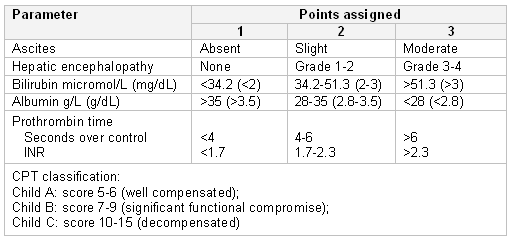
Model of End-Stage Liver Disease (MELD)
Electronically calculated from the serum bilirubin, sodium, creatinine, and clotting (INR and prothrombin time) by a specific computer programme.[67][68] [ MELDNa scores (for liver transplantation listing purposes, not appropriate for patients under age 12 years) (SI units) Opens in new window ] This is the classification system used for the allocation of livers for transplantation in the US.
Non-invasive tests
Serological and indirect markers of fibrosis have a good negative predictive value for cirrhosis, and are often used in community settings to risk-stratify patients with risk factors for liver disease. The American Association for the Study of Liver Diseases (AASLD) suggests a combination of imaging-based and blood-based techniques to detect significant fibrosis and advanced fibrosis, particularly in those undergoing initial fibrosis staining.[69]
Imaging-based techniques to detect fibrosis
Imaging-based tests may be preferentially incorporated into the initial fibrosis staging process owing to their higher accuracy over blood-based techniques. These tests are recommended for the identification of significant fibrosis, advanced fibrosis, and cirrhosis in adults with chronic hepatitis B and hepatitis C infections and in those with MASLD.[69] Imaging-based non-invasive testing may also be used in adults with alcohol-related liver disease or chronic cholestatic liver disease to detect advanced fibrosis or cirrhosis.
Either transient elastography or magnetic resonance elastography is recommended by the AASLD to stage fibrosis in adults with chronic liver disease.[69] The AASLD advises against using imaging-based tests as a standalone test to assess regression or progression of liver fibrosis.[69]
Transient elastography is an ultrasound-based technique for detecting hepatic fibrosis and cirrhosis without the need for liver biopsy.
As with non-invasive blood tests for fibrosis evaluation, transient elastography has best diagnostic performance in excluding liver cirrhosis. The accuracy falls in intermediate stages of fibrosis. The Society of Radiologists in Ultrasound recommends a low cut-off value to exclude significant fibrosis, and a high cut-off value to indicate compensated advanced chronic liver disease.[70] Meta-analyses and prospective studies of transient elastography report excellent diagnostic accuracy for the diagnosis of cirrhosis (independent of the underlying disease) and the identification of fibrosis in patients with recurrent hepatitis C infection after liver transplantation.[71][72][73][74][75][76][77][78][79]
As with transient elastography, acoustic radiation force impulse (ARFI) imaging employs ultrasound to perform elastography.
Magnetic resonance elastography is effective in measuring fibrosis, but its application is limited by cost, and it may not be possible if older metal prostheses are present in the patient. Staging of fibrosis may be possible using gadoxetic acid-enhanced MRI.[80]
FibroScan® is a non-invasive modality that helps quantify and stage hepatic steatosis and fibrosis (especially advanced fibrosis and cirrhosis) by measuring the degree of liver stiffness using vibration-controlled transient elastography.[81][82] It can distinguish normal liver or minimal fibrosis from cirrhotic livers.[82] FibroScan® can also be considered for the assessment of fibrosis or cirrhosis outside secondary and specialist care as it has a potential to detect liver disease earlier. It should be used in accordance with the national guidelines. The test should be recommended if it benefits people who lack adequate healthcare access (such as disabled people, people living in rural areas, or people from lower socio-economic backgrounds) and should be performed by trained operators.[82]
Blood-based techniques to detect fibrosis
Several fibrosis markers have been assessed. Some of these use combinations of routinely collected blood tests (e.g., NAFLD fibrosis score, fibrosis-4 [FIB-4], Enhanced Liver Fibrosis [ELF] test, AST to platelet ratio index [APRI], AST/ALT ratio). [ NAFLD Fibrosis Score Opens in new window ] Others use specific molecular markers of fibrogenesis (e.g., enhanced liver fibrosis). The utility of these markers is predominantly for excluding severe fibrosis, with normal values being reassuring. They do not perform well at differentiating intermediate stages of fibrosis. The AASLD recommends initial testing with APRI or FIB-4 markers to detect significant fibrosis, advanced fibrosis, or cirrhosis in adults with chronic hepatitis B and hepatitis C infections undergoing fibrosis staging prior to antiviral therapy.[83] The European Association for the Study of the Liver recommends that among non-invasive methods, the use of transient elastography has been mostly studied and seems to offer a higher diagnostic accuracy for the detection of cirrhosis in patients with chronic hepatitis B.[84]
Portal pressure assessment
Clinically significant portal hypertension is defined by a portal pressure gradient of ≥10 mmHg, which is also a predictor of clinical decompensation.[2] The gold standard method to assess portal pressure in patients with cirrhosis is hepatic venous pressure gradient.[2] Calculating hepatic venous pressure gradient is an invasive procedure and, as a result, it is not routinely used in all patients with liver cirrhosis and portal hypertension. In some patients with obesity or metabolic dysfunction-associated steatohepatitis (previously known as non-alcoholic steatohepatitis), hepatic venous pressure gradient may underestimate portal pressure.
Non-invasive assessment of clinically significant portal hypertension may be performed using a combination of LSM and platelet count.[2][85] An LSM of ≥20 kPa suggests clinically significant portal hypertension.[86] Clinically significant portal hypertension is present in all patients with varices.
Use of this content is subject to our disclaimer