Investigations
1st investigations to order
culture of ulcer swabs
Test
Diagnostic standard, despite relatively low sensitivity (53% to 92%).[54] Specialised media are required to optimise growth (MH-HB and/or GC-HgS); ideally, more than one type of media should be used to improve H ducreyi recovery.
Sample collection should ideally be from the base of the lesion without surface genital skin as swabs from the ulcer base have a higher yield than those from the bubo.[44][53]
Tissue is collected from the cleansed ulcer base and undermined margins with a Dacron, cotton, or calcium alginate swab. A needle and syringe should be used to aspirate purulent bubo material.
At room temperature, H ducreyi remains viable for only 2-4 hours on a swab and for only 24 hours on transport media; however, if the medium is kept at 4°C, H ducreyi will remain viable for up to 4 days.[63][67]
Culture should only be undertaken by laboratories that regularly perform testing for H ducreyi.[44]
Strains are catalase negative, are oxidase and beta-lactamase positive, and require X but not V factor for growth. Characteristically highly self-adherent and can be 'nudged' intact across the agar.[17][63][68]
Result
identification of Haemophilus ducreyi: colonies are non-spore-forming, non-mucoid; may be translucent to opaque with tan, yellowish, or yellowish-gray colouration
Haemophilus ducreyi polymerase chain reaction (PCR)
Test
Due to the difficulties with culture, PCR should be considered the test of choice where available.[53]
More sensitive and specific than other methods in multiple studies. Sensitivity as high as 98% and specificity >90%. Primarily used in reference labs.[56][57][58][59][60][61]
Sample collection should ideally be from the base of the lesion without surface genital skin as swabs from the ulcer base have a higher yield than those from the bubo.[44][53]
Tissue is collected from the cleansed ulcer base and undermined margins with a Dacron, cotton, or calcium alginate swab. A needle and syringe should be used to aspirate purulent bubo material. At room temperature, H ducreyi remains viable for only 2-4 hours on a swab.
Swabs should be placed in a dry, sterile tube for PCR testing. If the PCR specimen cannot be processed promptly, it should be stored frozen at -20°C to -70°C.
Result
positive
Gram stain of ulcer swabs
Test
May be useful, especially if the classical appearance of H ducreyi is seen.[Figure caption and citation for the preceding image starts]: Gram stain of Haemophilus ducreyiAdapted from G. Hammond, Public Health Image Library, CDC (1978) [Citation ends].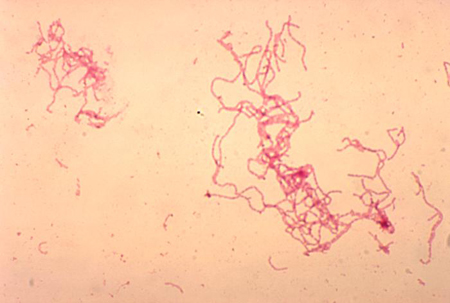
Sample collection should ideally be from the base of the lesion without surface genital skin as swabs from the ulcer base have a higher yield than those from the bubo.[44]
Tissue is collected from the cleansed ulcer base and undermined margins with a Dacron, cotton, or calcium alginate swab. A needle and syringe should be used to aspirate purulent bubo material.
Gram staining is not a reliable finding (sensitivity 5% to 63%; specificity 51% to 99%) because of the polymicrobial flora of most genital ulcers.[62]
Result
gram-negative coccobacilli or slender bacilli in railroad or chaining pattern (distinctive 'school of fish' arrangement)
syphilis testing
Test
To exclude syphilis as a differential diagnosis or co-infection.
The most common approach is to use a treponemal test as the initial serological test, followed by a non-treponemal test to confirm diagnosis and provide evidence of active disease or re-infection (i.e., a ‘reverse sequence screening algorithm’).[66] However, use of a traditional screening algorithm (initial investigation with a non-treponmeal test followed by a treponemal test) is also acceptable.
Dark-field microscopy of a lesion swab can provide a definitive diagnosis of syphilis, but it is not usually available outside of consultant settings.
See Syphilis infection.
Result
usually negative; positive in syphilis co-infection or prior syphilis infection
Herpes simple virus (HSV) PCR and viral cultures
Result
usually negative; positive in herpes co-infection
HIV test
Test
Chancroid is an important co-factor in HIV infection, and HIV testing should therefore be undertaken as part of the diagnostic work-up.[27] Local guidance for HIV testing varies.
See HIV infection.
Result
negative or positive
Investigations to consider
Haemophilus ducreyi serology
Haemophilus ducreyi antimicrobial sensitivity
Test
Not used routinely due to difficulty in culturing H ducreyi, lack of standardisation, and lack of interpretive guidelines.
Reserved for cases in which response to antibiotic therapy is poor.
Studies have shown in vitro resistance to tetracyclines, sulfonamides, penicillins, and amoxicillin/clavulanate. Most isolates remain susceptible to rifamycins, quinolones, macrolides, ceftriaxone and streptomycin.[17][26][64][65]
Can be done by agar dilution or E-test.[63]
Result
sensitivity to particular antibiotics
ulcer biopsy
Test
Rarely required.
A Tzanck smear is used to definitively diagnose or exclude herpes if infection is strongly suspected, and results of viral polymerase chain reaction and culture are inconclusive.[Figure caption and citation for the preceding image starts]: Tzanck test specimen showing multi-nucleated giant cells, indicating the presence of herpes virusAdapted from J. Miller, Public Health Image Library, CDC (1975) [Citation ends].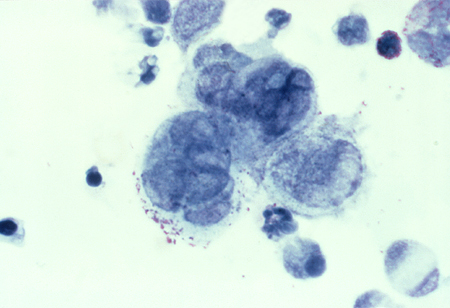
Also used to exclude squamous cell carcinoma.
Result
usually negative; Tzanck smear is positive in herpes co-infection
Emerging tests
immunofluorescence: direct or indirect Haemophilus ducreyi antigen testing
Test
Monoclonal antibodies are designed to detect lipo-oligosaccharide or HbA antigens.
Limited by cross-reactivity and not available commercially.
Compared with culture, direct fluorescent antibody testing has a sensitivity as high as 93% for ulcer specimens, but specificity is poor (63%).[2]
Result
positive for H ducreyi antigens
Use of this content is subject to our disclaimer