Approach
The main goals of treatment of spontaneous pneumothoraces are to remove the air from the pleural space and to decrease the likelihood of recurrence. If a tension pneumothorax is suspected, prompt intervention is required to decompress (needle or open) the involved hemithorax.[1]
The initial treatment includes observation with supplemental oxygen therapy, percutaneous aspiration of the air in the pleural space, and chest-tube thoracostomy, depending on the type and size of the pneumothorax.
[  ]
Video-assisted thoracoscopic surgery (VATS) or thoracotomy may be necessary to eliminate the site of the air leak if it remains persistent or if the patient has a recurrence.[24][34][53][54]
]
Video-assisted thoracoscopic surgery (VATS) or thoracotomy may be necessary to eliminate the site of the air leak if it remains persistent or if the patient has a recurrence.[24][34][53][54]
Pleurodesis is used to limit the likelihood of recurrence. It can be accomplished either by mechanical abrasion of the pleura under VATS or by introduction of a substance into the pleural space (i.e., talc) that irritates the pleural surfaces, with subsequent adhesion of the parietal and visceral pleura. The procedure of choice depends on patient characteristics and clinical circumstances.[24][52][53]
Several methods have been proposed for estimating the size of pneumothoraces on plain posterior-anterior chest radiographs. Unfortunately, each of these methods suffers from inaccuracies and/or lack of validation.[55] According to the British Thoracic Society (BTS) 2023 guidelines, the size of a pneumothorax is no longer an indication for invasive management, though it does dictate the safety of conducting an intervention. BTS now base their treatment recommendations on the clinical scenario and patient preferences.[24]
In the US, there are no recent guidelines on the management of pneumothorax and in clinical practice the size of pneumothorax continues to guide decisions regarding management; however, it is less important than the degree of clinical compromise.[56] Generally ≥2 cm laterally or apically on a chest x-ray (CXR) is considered sufficient size for intervention.[24] Pneumothoraces can be small (visible rim of <2 cm between the lung margin and the chest wall) or large (visible rim at least 2 cm between the lung margin and the chest wall).[24]
Tension pneumothorax
A tension pneumothorax is a medical emergency.
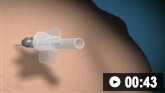
Decompression of a tension pneumothorax that is not secondary to trauma is accomplished by immediate insertion of a large-bore cannula (e.g., a 14-Fr angiocath) into the pleural space through the second intercostal space in the midclavicular line or the fourth or fifth intercostal space in the midaxillary line.[57] A "hiss" of air confirms the diagnosis. Intervention should not be delayed by awaiting radiographic confirmation of the tension pneumothorax. Point of care ultrasound (POCUS) can be useful in identifying lung point and a lack of lung sliding, which can be done rapidly at bedside to help guide patient care.
How to decompress a tension pneumothorax. Demonstrates insertion of a large-bore intravenous cannula into the fourth intercostal space in an adult.
If the tension pneumothorax is secondary to trauma, open thoracotomy for decompression is recommended if the expertise is available.[58] The Advanced Trauma Life Support guideline recommends using the fourth or fifth intercostal space in the midaxillary line as first-line if needle decompression is required.[59] Given common occurrence of hemothorax with pneumothorax in trauma, POCUS can help guide management, with chest tube placement preferentially in those situations to evacuate both air and blood.[60]
Primary spontaneous pneumothorax
Clinically stable patients who are experiencing a small primary spontaneous pneumothorax can be observed and treated conservatively with supplemental high-concentration (10 L/minute) oxygen and observation without invasive intervention.[24][53][54][61] The addition of high-concentration oxygen therapy has been shown to result in a fourfold increase in the rate of pneumothorax reabsorption during periods of oxygen supplementation.[62]
If the pneumothorax is large, needle aspiration is generally safe and effective. Often the procedure can be accomplished in the emergency department without admission to the hospital.[24][63][64] If intervention is deemed necessary, this can be accomplished by the placement of an intravenous cannula into the pleural space at the intersection of the midclavicular line and the second or third intercostal space. A large syringe can be used to withdraw air from the pleural space. Care must be taken not to allow air to gain access to the pleural space through the cannula. Coaching the patient to exhale while the syringe is detached from the cannula can prevent this from occurring. A stopcock attached to the cannula offers the advantage of sealing the pleural space from the atmosphere when the syringe is disconnected from the stopcock. Once no further air can be aspirated, the cannula should be removed and a CXR should be obtained.
Intercostal tube drainage may be superior to needle aspiration with respect to immediate resolution (low- to moderate-quality evidence), but data suggest no difference in recurrence at 1 year.[65][66] One randomized controlled trial of needle aspiration versus chest tube drainage in spontaneous pneumothorax (both primary and secondary) showed that needle aspiration was associated with shorter hospital stays and higher success rates when compared to chest tube drainage.[67] Needle aspiration remains a first-line treatment option for primary spontaneous pneumothorax because of the simplicity of the procedure and the reduced duration of hospitalization.[65] However, recent literature has demonstrated noninferiority of conservative management as long as there is close follow-up, therefore patient symptoms should guide therapy as opposed to arbitrary size cutoffs.[68] Conservative management may be considered if patients are asymptomatic with normal observations.[68][69][70] If aspiration fails, a chest tube or small-bore catheter should be inserted into the pleural space. Small-bore catheters can be attached to a one-way flutter valve and usually do not require negative pressure suction.[24][52]
Case series data shows that patients above 50 years old may respond differently to needle aspiration.[24] This is largely thought to be as a result of unrecognized underlying pulmonary disease in this age group. Therefore, it is recommended that patients above 50 years old, particularly those with a significant smoking history, should be treated with the assumption of an underlying respiratory disease (i.e., treated as a secondary spontaneous pneumothorax).[24]
There is insufficient evidence to make any recommendations on the best treatment method for pneumothorax and persistent air leak in adults.[24] Although there is no evidence to support the routine use of suction in the management of pneumothorax, in select patients it is thought to help cause the apposition of the visceral and parietal pleura, thereby promoting healing of the air leak.[52] Most suction devices have a water-filled chamber through which air is removed from the pleural space. A persistent air leak is easy to identify by the bubbling seen in the drain.
VATS with stapling of the air leak (or blebectomy if present) and pleurodesis may also be suitable in some instances. Compared with open pleurectomy for primary spontaneous pneumothoraces, VATS results in reductions in length of hospitalization and analgesic requirements for pain control. Recurrence rates, however, are higher following VATS pleurectomy than following open pleurectomy.[71][72]
Thoracoscopic wedge resection is an alternative procedure to stem the air leak should it persist. This is often performed together with mechanical pleurodesis to prevent recurrence of the pneumothorax. However, the addition of mechanical pleurodesis does not appear to reduce the rate of recurrence when compared with wedge resection alone.[73] In addition, patients receiving wedge resection and mechanical pleurodesis have higher rates of intraoperative bleeding and postoperative pleural drainage rates.[73] Rather than performing mechanical pleurodesis following wedge resection, there is some evidence to suggest visceral pleural coverage of the staple line with absorbable cellulose mesh and fibrin glue is equivalent to mechanical pleurodesis without the associated potential complications.[74] In nonoperative candidates, autologous blood patch, talc slurry via chest tube (or other sclerosant) pleurodesis, or endobronchial one-way valve placement can be considered.[75][76][77]
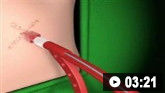
How to insert an intercostal (chest) drain using the Seldinger technique. Video demonstrates: how to identify a safe site for insertion; use of an introducer needle, guidewire, dilators, and intercostal drain; how to confirm drain position; and postprocedure care.
Secondary spontaneous pneumothorax
The size of a secondary spontaneous pneumothorax may not correlate well with the clinical manifestations as they are dependent on the extent of the underlying disease and the patient’s respiratory reserve. In general, the clinical symptoms associated with secondary spontaneous pneumothoraces are more severe than those associated with primary spontaneous pneumothoraces; therefore, these patients typically require hospitalization.[24] Moreover, the recurrence rate in patients with pulmonary diseases is somewhat higher than that of primary spontaneous pneumothorax.
In clinically stable patients who are experiencing a secondary spontaneous pneumothorax that is too small for a chest tube to be placed safely (<1 cm), treatment should include supplemental high-concentration (10 L/minute) oxygen, and expectant observation.[62] Oxygen should be used with caution in patients with chronic lung disease and hypercapnic respiratory failure (e.g., COPD).
In patients with moderately-sized pneumothoraces (1 cm to 2 cm), needle aspiration may be attempted. If needle aspiration fails to reduce the size of the pneumothorax significantly (<1 cm), then a chest tube or small-bore catheter should be placed.[24]
In symptomatic patients with high-risk characteristics, a chest drain can be inserted.[24] Most patients with secondary spontaneous pneumothoraces will require a chest tube or small-bore catheter given poor healing associated with underlying lung disease.
Thoracic surgery should be considered at initial presentation if recurrence prevention is deemed important (e.g., patients presenting with tension pneumothorax, or those in high-risk occupations).[24]
If the pneumothorax fails to resolve despite the above treatment, the patient may require VATS with stapling of the air leak and pleurodesis. This is more effective than chemical pleurodesis. However, the perioperative morbidity and mortality of VATS in patients with secondary spontaneous pneumothorax may be prohibitively high.[24][52][53] In view of the significant risk of morbidity and mortality after VATS or open thoracotomy, less invasive measures may be tried, especially in patients with severe pulmonary disease, whether due to COPD, cystic fibrosis, or another lung disorder. In nonoperative candidates, autologous blood patch, talc slurry via chest tube (or other sclerosant) pleurodesis, or endobronchial one-way valve placement can be considered.[75][76][77] Talc pleurodesis should also be considered on the first episode of pneumothorax in high-risk patients in whom repeat pneumothorax would be hazardous (e.g., severe COPD).[24]
Subsequent interventions are aimed at preventing recurrences. In general, the chest tube should remain in place until a procedure is performed to prevent recurrent pneumothorax.[24][52][53]
While all patients with a secondary spontaneous pneumothorax should be considered for a preventive intervention, patients who are possible lung transplantation candidates require special consideration. Diffuse pleurodesis with either VATS or intrapleural chemical instillation should be avoided in patients with cystic fibrosis or alpha-1 antitrypsin deficiency, and in younger patients with smoking-related COPD who are being considered for lung transplant. Previous diffuse pleurodesis results in a more difficult and bloody dissection during the lung transplantation procedure. Conservative measures and observation or VATS without directed mechanical abrasion are preferred in this subset of patients.
Chemical pleurodesis may have a role in reducing recurrence of spontaneous pneumothorax following initial surgical intervention.[24] One systematic review of 50 abstracts covering a mixed population of both secondary and primary spontaneous pneumothorax reported that post-surgical or post-thoracoscopic chemical pleurodesis (talc or minocycline) was associated with low rates of pneumothorax recurrence (0% to 3.2% and 2.5% to 10.2%, respectively). The review reported higher rates of pneumothorax recurrence with chest drainage only or chemical pleurodesis via chest drain (26.1% to 50.1% and 13% to 18.2%, respectively). These findings are difficult to generalize as the number of randomized trials or comparative studies evaluating each agent is limited.[82]
How to insert an intercostal (chest) drain using the Seldinger technique. Video demonstrates: how to identify a safe site for insertion; use of an introducer needle, guidewire, dilators, and intercostal drain; how to confirm drain position; and postprocedure care.
Catamenial pneumothorax
The acute treatment of catamenial pneumothorax should include hormonal treatment or surgery by VATS.[24]
Some patients with catamenial pneumothorax also develop a hemothorax, resulting in a hemopneumothorax as a complication of their thoracic endometriosis. The blood in the pleural space has typically required a large-bore tube thoracostomy drainage, though hemothoraces can likely be managed with smaller-bore tubes.[83] Because patients with catamenial pneumothoraces are typically young and without underlying parenchymal lung disease, high-concentration oxygen can be administered without fear of hypercapnic respiratory failure.
The long-term treatment for catamenial pneumothorax is suppression of the ectopic endometrium by interfering with ovarian estrogen secretion. This can be accomplished with oral contraceptives, gonadotropin-releasing hormone analogs, progestins, and danazol. Many patients who experience catamenial pneumothoraces will not suffer recurrences as long as ovulation and menstruation are suppressed.[84]
If the patient cannot take ovulation-suppressing medications, wishes to discontinue this therapy to become pregnant, or fails hormonal manipulation, then an invasive procedure to prevent a recurrence of the catamenial pneumothorax should be considered. VATS or open thoracotomy can be performed. The pleura should be inspected for endometrial implants and the diaphragm examined for perforations. The implants should be excised and diaphragmatic defects repaired. Chemical or mechanical pleurodesis should also be undertaken to prevent recurrence.
Traumatic pneumothorax
First-line treatment involves percutaneous needle aspiration. If aspiration fails or the pneumothorax is large, a chest tube placement is usually required.
A hemothorax may accompany and/or complicate a traumatic pneumothorax. The presence of a hemothorax necessitates chest tube placement.[24] If bleeding continues, exploration of the thoracic cavity may be necessary to achieve hemostasis.
If the lung fails to re-expand or there is persistent air leakage after 72 hours, the patient is likely to require VATS or thoracotomy.[44]
How to insert an intercostal (chest) drain using the Seldinger technique. Video demonstrates: how to identify a safe site for insertion; use of an introducer needle, guidewire, dilators, and intercostal drain; how to confirm drain position; and postprocedure care.
Pneumothorax ex vacuo
Patients with pneumothorax ex vacuo should be given high-concentration oxygen (as long as they are not at risk of hypercapnic respiratory failure), but may require bronchoscopy to relieve the endobronchial obstruction. Tube thoracostomy is not indicated for both endobronchial obstruction, as well as nonexpandable lung as there is no visceral pleural injury or ongoing air leak.[4]
How to decompress a tension pneumothorax. Demonstrates insertion of a large-bore intravenous cannula into the fourth intercostal space in an adult.
How to insert an intercostal (chest) drain using the Seldinger technique. Video demonstrates: how to identify a safe site for insertion; use of an introducer needle, guidewire, dilators, and intercostal drain; how to confirm drain position; and postprocedure care.
How to insert an intercostal (chest) drain using the open technique. Video demonstrates: tube selection, how to identify the site for drain insertion, how to make the correct incision, how to insert the intercostal drain, how to secure the drain, and postprocedure care.
Use of this content is subject to our disclaimer