Approach
The main goal of initial treatment is to control the bleeding, prevent any complication relating to it, eliminate the underlying cause whenever possible, and to identify patients at risk for rebleeding or those who will need admission to the hospital for further treatment.
Initial resuscitation
Patients who are actively bleeding and/or with multiple medical comorbidities should be attended to as an emergency. Patients in shock should be managed in a critical care setting wherever possible. The initial diagnostic evaluation should have included an assessment of hemodynamic stability and resuscitation if necessary, implementing the ABC (airway, breathing, and circulation) approach.[42]
Bilateral, peripheral, or central intravenous access is important to maintain adequate fluid replacement (crystalloids), which is the single most important aspect of initial resuscitation. Blood transfusion (packed RBCs) may be necessary in certain cases (ongoing bleeding, or low hemoglobin [Hb] at presentation). Guidelines differ in exact thresholds. The American College of Gastroenterologists recommends transfusion at Hb <7 g/dL, or <8 g/dL in patients with cardiovascular disease (CVD), fluid resuscitation-related hemodilution, or multiple comorbidities.[39] The International Consensus Group was concerned about undiagnosed CVD and suggests a slightly higher transfusion threshold Hb of 8 g/dL.[40] A target of Hb ≥10 g/dL should be aimed for in patients with CVD, while a lower level of Hb 7-9 g/dL is considered appropriate for patients without CVD.[1] Similarly, although it does not specify ranges, the American College of Chest Physicians recommends a restrictive transfusion strategy over a permissive transfusion strategy in critically ill patients, notably including those with acute gastrointestinal bleeding, but in critically ill patients with acute coronary syndrome, they suggest against a restrictive transfusion strategy.[41] Platelets and coagulation factors should be given in certain circumstances. Patients on antiplatelet treatment should not receive platelet transfusions.[59] Diagnostic assessment is performed simultaneously.
To decompress the stomach and allow gastric lavage, a nasogastric or orogastric tube could be placed carefully in patients with ongoing bleeding or those suspected of having concomitant upper gastrointestinal (GI) bleeding sources, such as peptic ulcer disease, esophageal varices, and Dieulafoy lesions, although in practice this is rarely performed. One retrospective propensity-matched analysis of 632 US veterans with GI bleeding showed that nasogastric lavage did not affect mortality, transfusions, length of hospital stay, or surgery, but was associated with earlier time to endoscopy.[60] Many guidelines do not make any recommendations regarding the placement of nasogastric or orogastric tubes.[39][40][43]
Elective endotracheal intubation should be considered in patients with ongoing hematemesis or altered respiratory or mental status. Elective endotracheal intubation will protect the airway and facilitate endoscopy.
Electrolyte abnormalities should be corrected with appropriate replacement fluids.
Prolonged prothrombin time/international normalized ratio (PT/INR) should be corrected with fresh frozen plasma and/or vitamin K (phytonadione) in all patients except for those on warfarin treatment.[59] Prothrombin complex concentrate can be considered in patients on warfarin with a life-threatening GI bleed or those with a supratherapeutic INR substantially exceeding the therapeutic range. Prothrombin complex concentrate can also be considered for patients in whom massive blood transfusion is undesirable because of its effect on coagulopathy or dilution of blood components.[59] Patients on direct oral anticoagulants (DOACs) should not routinely be given prothrombin complex concentrate; those on dabigatran should not routinely receive idarucizumab, and those on rivaroxaban or apixaban should not routinely receive recombinant coagulation factor Xa (andexanet alfa). These recommendations are based on limited evidence of benefit. However, reversal of anticoagulation with these agents may be considered in hospitalized patients with a life-threatening GI bleed who have taken a DOAC within the past 24 hours.[59]
Patients on aspirin for secondary prevention should continue with this; if aspirin is stopped, it should be restarted on the day hemostasis is confirmed endoscopically.[59]
Several risk scoring systems have been developed and validated, but each has been shown to perform most accurately to predict particular outcomes: for example, mortality, risk of rebleeding, need for transfusion, or the need for surgical or endoscopic therapy. In summary, the major international, American, and European upper GI bleeding guidelines only suggest the Glasgow-Blatchford bleeding score (GBS) to identify, with high certainty, very low-risk patients who can be safely managed with outpatient endoscopy.[39][40][43] The GBS (pre-endoscopy score) is calculated using the following parameters: blood urea nitrogen, Hb, systolic blood pressure, pulse, melena, history or evidence of liver disease, and coronary artery disease.[38][44][45] Patients with a score of 0-1 are classified as very low-risk, which indicates a ≤1% false negative rate for requiring in-hospital interventions or death.[39][40][43] Patients with a score of ≥2 should be admitted promptly and receive an endoscopy within 24 hours.[1][39] [ Blatchford score for gastrointestinal bleeding Opens in new window ]
Pharmacologic treatment
Initial pharmacologic management may include an antiemetic, erythromycin, and a proton-pump inhibitor (PPI).
Erythromycin
Erythromycin is thought to stimulate motilin receptors, with subsequent increase in stomach contraction, which may help to mobilize gastric clots and allow better esophagogastroduodenoscopy (EGD) assessment.[39][51][52][53][54]
In one systematic review conducted by the American College of Gastroenterologists, erythromycin reduced the number of repeat endoscopies, and the length of hospitalizations by at least 1 day.[39]
Promotility agents should not be used routinely before endoscopy to increase the diagnostic yield.[1][40] They may be considered if there is suspected large volume food or blood in the stomach.[61]
There are few data to support the use of metoclopramide.[39]
Antisecretory therapy
The role of PPIs in MWT is not clear. Guidelines have made conflicting recommendations regarding the administration of PPIs prior to diagnostic EGD, when the exact cause of the bleeding remains unclear.[39][40] [
 ]
]
Given the lack of certainty around the data, it is suggested that local protocols are followed. Some experts still suggest pre-endoscopy PPIs to downgrade any high-risk lesions particularly when early endoscopy cannot be performed, or if contraindications to endoscopic evaluation exist.[50] In the experience of this author, the benefits of giving PPIs prior to endoscopy outweigh potential harms and PPIs are widely administered for nonvariceal upper GI bleeding.
PPI treatment started before endoscopy may reduce the need for endoscopic treatment in patients with nonvariceal bleeding.[62] [
 ]
However, the benefit of using a PPI to prevent rebleeding, and reduce mortality or need for surgery, has not been demonstrated in patients with nonvariceal bleeding.[62][63][64]
[
]
However, the benefit of using a PPI to prevent rebleeding, and reduce mortality or need for surgery, has not been demonstrated in patients with nonvariceal bleeding.[62][63][64]
[  ]
In patients with bleeding ulcers, intravenous bolus followed by continuous infusion has been shown to effectively decrease rebleeding in patients who have had successful endoscopic therapy, but these data are difficult to interpret in light of MWT.[65]
]
In patients with bleeding ulcers, intravenous bolus followed by continuous infusion has been shown to effectively decrease rebleeding in patients who have had successful endoscopic therapy, but these data are difficult to interpret in light of MWT.[65]If given pre-endoscopy, PPIs are generally administered via the intravenous route. Common PPIs used for actively bleeding lesions include intravenous pantoprazole or esomeprazole. Oral PPI therapy should be considered in those not actively bleeding.[63][64] Oral esomeprazole or rabeprazole can be used.
H2 antagonists can be considered as an alternative therapy to PPIs when PPIs are contraindicated. However, treatment with PPIs achieves more profound and sustained acid suppression than H2 antagonists.[66][67] For actively bleeding lesions, intravenous famotidine can be given followed by oral famotidine. For non-actively bleeding lesions, oral famotidine or cimetidine can be given.
Antiemetics
An antiemetic is useful for controlling nausea and vomiting, which may be a cause or an aggravating factor in patients with MWT, and is administered pre-endoscopy, if needed.
Common antiemetics recommended include promethazine, ondansetron, prochlorperazine, and trimethobenzamide.
Endoscopic treatment
EGD is the procedure of choice for patients with nonvariceal upper GI bleeding.[50][57] It is usually performed after resuscitation, stabilization, and pharmacologic treatment is given.
Several endoscopic modalities are effective for treating high-risk bleeding lesions such as an oozing or an actively bleeding/spurting tear. The choice of methods used will depend on the endoscopist's familiarity, experience, and training, and on equipment availability.
Studies have not demonstrated clear superiority of one technique over others.[68][69][70] However, dual therapy is superior to single therapy such as epinephrine (adrenaline) injection. Complete endoscopic exam is recommended because coexisting lesions are commonly associated with MWT.
Different modalities of treatment include the following:
Injection therapy is commonly used in the treatment of ulceration causing upper GI bleeding, in combination with thermal or mechanical therapy. Its role in the management of MWT bleeding has been studied in a small series.[71][72] Epinephrine injection is generally the most common agent used: this agent stops or reduces the bleeding rate by vasoconstriction and tamponade. It is injected around or into the bleeding point. Sclerosant agents, such as ethanolamine and alcohol, have been described in the literature; however, they are rarely used in practice.[73]
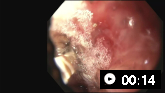
[Figure caption and citation for the preceding image starts]: Actively bleeding tear appears as a red longitudinal defect with normal surrounding mucosaFrom the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].
 [Figure caption and citation for the preceding image starts]: Epinephrine is injected intravenously next to Mallory-Weiss tearFrom the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].
[Figure caption and citation for the preceding image starts]: Epinephrine is injected intravenously next to Mallory-Weiss tearFrom the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].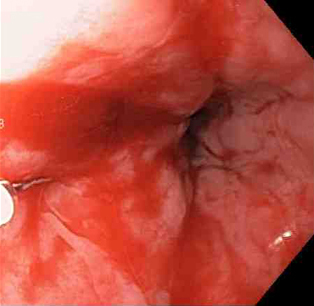 [Figure caption and citation for the preceding image starts]: Mallory-Weiss tear after epinephrine injection (the bleeding has stopped, allowing better visualization of the lesion)From the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].
[Figure caption and citation for the preceding image starts]: Mallory-Weiss tear after epinephrine injection (the bleeding has stopped, allowing better visualization of the lesion)From the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].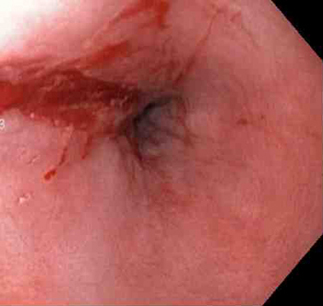
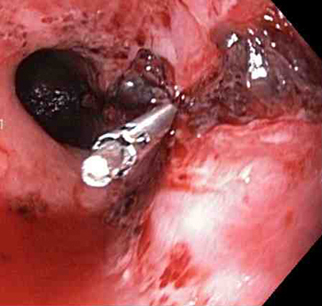
Through-the-scope clips (TTSCs), alone or in combination with epinephrine injection, can effectively control actively bleeding lesions, and the clips are as effective and safe as other methods.[74][75][76] In the author's experience, this is the technique of choice in a patient with an actively bleeding tear or laceration. The application of TTSCs and an endoloop in the esophagus has also been described as a method of closure of large MWTs.[77][Figure caption and citation for the preceding image starts]: A through-the-scope clip deployed in the center of the lesion (no previous epinephrine was infused in this case)From the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].
 [Figure caption and citation for the preceding image starts]: Nonbleeding adherent clotFrom the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].
[Figure caption and citation for the preceding image starts]: Nonbleeding adherent clotFrom the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends]. [Figure caption and citation for the preceding image starts]: Three through-the-scope clips deployed to complete closure of the mucosal defectFrom the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].
[Figure caption and citation for the preceding image starts]: Three through-the-scope clips deployed to complete closure of the mucosal defectFrom the collection of Juan Carlos Munoz, MD, University of Florida [Citation ends].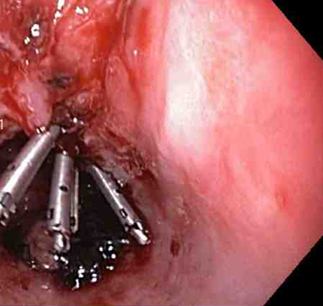
Over-the-scope clips (OTSC) are available from several vendors and are larger, often permanent clips that have been shown to be effective in treating a wide variety of nonvariceal upper GI bleeding sources, including MWT.[78][79][80]
Various topical hemostatic agents that promote hemostasis have been shown to be a promising rescue therapy, or adjunct to conventional methods for treating upper GI bleeding including MWT.[1][40][81][82][83] They can be administered through the upper endoscope during EGD.[84][85][86]
Thermocoagulation therapy (also known as thermal therapy) includes multipolar electrocoagulation (MPEC), heater probe, and argon plasma coagulation (APC). It may be used as a single therapy; however, it is most commonly used in combination with injection therapy. Thermal therapies including MPEC or heater probe are reserved for actively bleeding lesions. They are often successful and safely given by experienced operators; however, contact thermal therapy should be used with caution when used in the esophagus for a bleeding MWT because of the risk of perforation.[1][87][88] The evidence for APC in this scenario is limited. However, the lack of contact between the catheter and the tissue results in a superficial hemostasis, reducing unwanted tissue damage and perforation, making this technique attractive in certain cases in which other methods of treatment are not available.[89]
Mallory Weiss tear following cauterization with a bipolar probeFrom the personal collection of Douglas Adler; used with permission
Endoscopic band ligation (EBL) has been described in case reports and small controlled trials, but in the experience of this author, is not commonly used for treatment of MWT. The mechanism is similar to that described for variceal band ligation: the affected mucosa is suctioned into the ligating cup device, after which a rubber band is deployed. EBL is simple and safe.[90][91][92] For most patients, EBL is used as a single therapy in which the bleeding point is aspirated and ligated. In some unusual cases, in which the bleeding point is not easily localized, EBL has been used as a combination therapy with epinephrine. Epinephrine injection may decrease or slow down the bleeding and help to localize the bleeding point.
Surgery
Surgery, which in practice is very rarely warranted in patients with MWT, should be reserved for situations where endoscopic hemostasis of bleeding has failed or transmural esophageal perforation has occurred.[57] Laparoscopic over-sewing of the laceration at the gastroesophageal junction can be readily performed under video control with endoscopic guidance with excellent results.[93]
Angiography is indicated for massive bleeding when EGD and surgery are not readily available, or when patients have an absolute contraindication to surgery or endoscopic therapy.[94][95]
Angiography therapy can be performed using either of the following methods:
Infusion of a vasoconstrictor agent such as vasopressin into the superior mesenteric artery, left gastric artery, or by direct infusion into a vessel that leads to a bleeding artery.[96]
Transcatheter embolization with an artificial material such as gelfoam or less often with the patient's own clotted blood.[97][98]
Use of this content is subject to our disclaimer