The majority of women with uterine fibroids are asymptomatic and require no further investigation or treatment unless rapid growth is noted or there are other reasons to suspect pelvic malignancy.[2]Bradley LD, Falcone T. Myomectomy. In: Handa VL, Van Le L, eds. Te Linde's operative gynecology. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2019:325-47.[9]American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 228: management of symptomatic uterine leiomyomas. Jun 2021 [internet publication].
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
The goals of treatment should be centred on the safe and effective amelioration of symptoms, and minimisation of persistence or recurrence of symptoms, while addressing future fertility desires and patient wishes regarding uterine preservation.
For the purpose of treatment selection, patients may be subgrouped into those who desire uterine preservation for future fertility or other considerations, and those for whom uterine preservation is not a consideration. Unfortunately, there is a lack of good-quality evidence in the literature for efficacy of most interventions for the treatment of symptomatic uterine fibroids.[82]Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids: comparative effectiveness review No. 195. AHRQ publication no. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017.
https://effectivehealthcare.ahrq.gov/products/uterine-fibroids/research-2017
The US Food and Drug Administration (FDA) issued a safety communication cautioning against the use of laparoscopic power morcellation during hysterectomy or myomectomy for the treatment of women with uterine fibroids. This is because power morcellation carries a risk of spreading undetected uterine sarcoma, with resultant worsening prognosis.[83]US Food and Drug Administration. FDA updated assessment of the use of laparoscopic uterine power morcellators to treat uterine fibroids. Dec 2017 [internet publication].
https://www.fda.gov/downloads/medicaldevices/productsandmedicalprocedures/surgeryandlifesupport/ucm584539.pdf
[84]American College of Obstetricians and Gynecologists. Committee opinion no. 822: uterine morcellation for presumed leiomyomas. Mar 2021 [internet publication].
https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2021/03/uterine-morcellation-for-presumed-leiomyomas
Uterine sarcoma is a rare medical condition, particularly in the setting of long-standing uterine fibroids as present in the majority of patients.
Medical therapies
Among patients requesting uterine preservation, several medical therapies have been employed and virtually all salutary effects cease on discontinuation of therapy. Medical therapies for symptomatic fibroids are reversible (as are their salutary effects) and therefore fertility-sparing.[9]American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 228: management of symptomatic uterine leiomyomas. Jun 2021 [internet publication].
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
These agents are effective in temporarily ameliorating the major symptoms associated with uterine fibroids such as bulk-related complaints and heavy bleeding.[85]Tropeano G, Amoroso S, Scambia G. Non-surgical management of uterine fibroids. Hum Reprod Update. 2008 May-Jun;14(3):259-74.
https://academic.oup.com/humupd/article/14/3/259/684493
http://www.ncbi.nlm.nih.gov/pubmed/18344356?tool=bestpractice.com
Nevertheless, adverse effects associated with prolonged use limit their utility to the short term (3 to 6 months).[86]Cheng MH, Wang PH. Uterine myoma: a condition amenable to medical therapy? Expert Opin Emerg Drugs. 2008 Mar;13(1):119-33.
http://www.ncbi.nlm.nih.gov/pubmed/18321152?tool=bestpractice.com
While oral contraceptives may be used when menstrual bleeding associated with fibroids is a concern, combined oral contraceptives significantly reduce blood loss after long-term use. But there is no general consensus on benefits and effect on fibroid growth.[87]Sayed GH, Zakherah MS, El-Nashar SA, et al. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynaecol Obstet. 2011 Feb;112(2):126-30.
https://www.doi.org/10.1016/j.ijgo.2010.08.009
http://www.ncbi.nlm.nih.gov/pubmed/21092958?tool=bestpractice.com
Gonadotrophin-releasing hormone (GnRH) agonists (e.g., leuprorelin) rapidly induce a low oestrogen state with amenorrhoea and result in fibroid shrinkage by 35% to 65%, with return to pretreatment size shortly following cessation of treatment.[88]Olive DL, Lindheim SR, Pritts EA. Non-surgical management of leiomyoma: impact on fertility. Curr Opin Obstet Gynecol. 2004 Jun;16(3):239-43.
http://www.ncbi.nlm.nih.gov/pubmed/15129053?tool=bestpractice.com
The accompanying vasomotor symptoms and bone loss seen with prolonged use of these agents make them impractical for use except if used preoperatively to reverse anaemia and stabilise haematocrit before myomectomy or hysterectomy.[72]Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004 Aug;104(2):393-406.
http://www.ncbi.nlm.nih.gov/pubmed/15292018?tool=bestpractice.com
[88]Olive DL, Lindheim SR, Pritts EA. Non-surgical management of leiomyoma: impact on fertility. Curr Opin Obstet Gynecol. 2004 Jun;16(3):239-43.
http://www.ncbi.nlm.nih.gov/pubmed/15129053?tool=bestpractice.com
The use of 'add-back therapy' with hormone replacement therapy may avoid the vasomotor side effects and bone effects of GnRH analogue therapy.[89]Morris EP, Rymer J, Robinson J, et al. Efficacy of tibolone as "add-back therapy" in conjunction with a gonadotropin-releasing hormone analogue in the treatment of uterine fibroids. Fertil Steril. 2008 Feb;89(2):421-8.
http://www.ncbi.nlm.nih.gov/pubmed/17572410?tool=bestpractice.com
[90]Lethaby AE, Vollenhoven BJ. An evidence-based approach to hormonal therapies for premenopausal women with fibroids. Best Pract Res Clin Obstet Gynaecol. 2008 Apr;22(2):307-31.
http://www.ncbi.nlm.nih.gov/pubmed/17905660?tool=bestpractice.com
Mifepristone is an antiprogestogen that has been shown to shrink fibroids by nearly 50% over a 6-month period and improve symptoms related to the fibroid. Although no bone loss is seen with this treatment, vasomotor symptoms and, more importantly, development of endometrial hyperplasia greatly limits the utility of this treatment.
[  ]
What are the effects of selective progesterone receptor modulators (SPRMs) for premenopausal women with uterine fibroids?/cca.html?targetUrl=http://cochraneclinicalanswers.com/doi/10.1002/cca.1784/fullShow me the answer It can cause transient elevations in transaminases.[91]Chwalisz K, Garg R, Brenner R, et al. Role of nonhuman primate models in the discovery and clinical development of selective progesterone receptor modulators (SPRMs). Reprod Biol Endocrinol. 2006;4 Suppl 1:S8.
https://www.doi.org/10.1186/1477-7827-4-S1-S8
http://www.ncbi.nlm.nih.gov/pubmed/17118172?tool=bestpractice.com
[92]Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci. 2012 Apr;19(4):339-53.
https://www.doi.org/10.1177/1933719111432867
http://www.ncbi.nlm.nih.gov/pubmed/22378865?tool=bestpractice.com
]
What are the effects of selective progesterone receptor modulators (SPRMs) for premenopausal women with uterine fibroids?/cca.html?targetUrl=http://cochraneclinicalanswers.com/doi/10.1002/cca.1784/fullShow me the answer It can cause transient elevations in transaminases.[91]Chwalisz K, Garg R, Brenner R, et al. Role of nonhuman primate models in the discovery and clinical development of selective progesterone receptor modulators (SPRMs). Reprod Biol Endocrinol. 2006;4 Suppl 1:S8.
https://www.doi.org/10.1186/1477-7827-4-S1-S8
http://www.ncbi.nlm.nih.gov/pubmed/17118172?tool=bestpractice.com
[92]Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci. 2012 Apr;19(4):339-53.
https://www.doi.org/10.1177/1933719111432867
http://www.ncbi.nlm.nih.gov/pubmed/22378865?tool=bestpractice.com
The levonorgestrel intrauterine contraceptive device significantly decreases bleeding in women with fibroid-associated heavy menstrual bleeding.[93]Grigorieva V, Chen-Mok M, Tarasova M, et al. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003 May;79(5):1194-8.
http://www.ncbi.nlm.nih.gov/pubmed/12738516?tool=bestpractice.com
[94]Varma R, Sinha D, Gupta JK. Non-contraceptive uses of levonorgestrel-releasing hormone system (LNG-IUS): a systematic enquiry and overview. Eur J Obstet Gynecol Reprod Biol. 2006 Mar 1;125(1):9-28.
http://www.ncbi.nlm.nih.gov/pubmed/16325993?tool=bestpractice.com
It is recommended as a first-line treatment for women with heavy menstrual bleeding associated with fibroids less than 3 cm in diameter that are not causing distortion of the uterine cavity.[61]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
[Evidence C]ed9b9e12-da18-4dd4-9e60-a91e56892f9fguidelineCWhat are the effects of levonorgestrel intrauterine device contraceptive devices as treatment for women with heavy menstrual bleeding associated with fibroids?[61]National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. May 2021 [internet publication].
https://www.nice.org.uk/guidance/ng88
Gonadotrophin-releasing hormone antagonists (e.g., elagolix, relugolix) have been shown to reduce heavy menstrual bleeding in women with uterine fibroids. These drugs are given in combination with hormonal 'add-back therapy'. The oral GnRH antagonist elagolix has been shown to significantly reduce heavy menstrual bleeding in women with fibroids.[95]Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020 Jan 23;382(4):328-40.
https://www.nejm.org/doi/full/10.1056/NEJMoa1904351
http://www.ncbi.nlm.nih.gov/pubmed/31971678?tool=bestpractice.com
Low-dose add-back regimens substantially reduced flushing associated with elagolix treatment.[95]Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020 Jan 23;382(4):328-40.
https://www.nejm.org/doi/full/10.1056/NEJMoa1904351
http://www.ncbi.nlm.nih.gov/pubmed/31971678?tool=bestpractice.com
[96]Archer DF, Stewart EA, Jain RI, et al. Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study. Fertil Steril. 2017 Jul;108(1):152-160.e4.
https://www.doi.org/10.1016/j.fertnstert.2017.05.006
http://www.ncbi.nlm.nih.gov/pubmed/28579415?tool=bestpractice.com
Elagolix (in a combination formulation with estradiol and noresthisterone) is approved by the FDA for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women.[95]Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020 Jan 23;382(4):328-40.
https://www.nejm.org/doi/full/10.1056/NEJMoa1904351
http://www.ncbi.nlm.nih.gov/pubmed/31971678?tool=bestpractice.com
Relugolix is another oral GnRH antagonist. It is approved (also in a combination formulation with estradiol and noresthisterone) by the FDA and the European Medicines Agency (EMA) for the management of symptoms associated with uterine fibroids in premenopausal women. Approval was supported by several phase 3 clinical trials that showed the drug was effective in reducing menstrual blood loss and was well tolerated.[97]Barra F, Seca M, Della Corte L, et al. Relugolix for the treatment of uterine fibroids. Drugs Today (Barc). 2019 Aug;55(8):503-512.
https://www.doi.org/10.1358/dot.2019.55.8.3020179
http://www.ncbi.nlm.nih.gov/pubmed/31461087?tool=bestpractice.com
[98]Osuga Y, Enya K, Kudou K, et al. Oral gonadotropin-releasing hormone antagonist relugolix compared with leuprorelin injections for uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2019 Mar;133(3):423-33.
https://www.doi.org/10.1097/AOG.0000000000003141
http://www.ncbi.nlm.nih.gov/pubmed/30741797?tool=bestpractice.com
[99]Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021 Feb 18;384(7):630-42.
https://www.doi.org/10.1056/NEJMoa2008283
http://www.ncbi.nlm.nih.gov/pubmed/33596357?tool=bestpractice.com
Non-steroidal anti-inflammatory drugs have been tried empirically in the medical management of excessive bleeding, dysmenorrhoea, and pelvic pain. These agents significantly reduce blood loss and improve pain relief compared with placebo, but are less effective at decreasing blood loss compared with the levonorgestrel intrauterine device or tranexamic acid.[100]Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019 Sep 19;9:CD000400.
https://www.doi.org/10.1002/14651858.CD000400.pub4
http://www.ncbi.nlm.nih.gov/pubmed/31535715?tool=bestpractice.com
[101]Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991 Mar;164(3):879-83.
https://www.doi.org/10.1016/s0002-9378(11)90533-x
http://www.ncbi.nlm.nih.gov/pubmed/1900665?tool=bestpractice.com
Tranexamic acid is an oral anti-fibrinolytic agent that significantly reduces menstrual blood loss compared with placebo and might reduce heavy menstrual bleeding in women with fibroids. A higher rate of fibroid necrosis was reported in patients who received tranexamic acid compared with untreated patients in one study.[102]Ip PP, Lam KW, Cheung CL, et al. Tranexamic acid-associated necrosis and intralesional thrombosis of uterine leiomyomas: a clinicopathologic study of 147 cases emphasizing the importance of drug-induced necrosis and early infarcts in leiomyomas. Am J Surg Pathol. 2007 Aug;31(8):1215-24.
http://www.ncbi.nlm.nih.gov/pubmed/17667546?tool=bestpractice.com
Side effects of tranexamic acid treatment include pelvic pain and fever, but more qualified studies are required to establish the therapeutic efficacy of tranexamic acid in women with symptomatic uterine fibroids, and to better define the drug's role in relation to fibroid type, size, and location.[103]Peitsidis P, Koukoulomati A. Tranexamic acid for the management of uterine fibroid tumors: a systematic review of the current evidence. World J Clin Cases. 2014 Dec 16;2(12):893-8.
https://www.wjgnet.com/2307-8960/full/v2/i12/893.htm
http://www.ncbi.nlm.nih.gov/pubmed/25516866?tool=bestpractice.com
Myomectomy to preserve fertility
Conservative surgical treatments that also allow for uterine preservation are also available.
The only surgical procedure that preserves fertility and effectively ameliorates fibroid-related symptoms is myomectomy. For women with poor prior reproductive outcome or infertility in whom the only finding is a distorted uterine cavity from the presence of one or more uterine fibroids (most commonly submucous fibroids), myomectomy can promote both fertility and successful pregnancy outcome.
To be applicable for myomectomy, patients should have one or more of the following:[72]Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004 Aug;104(2):393-406.
http://www.ncbi.nlm.nih.gov/pubmed/15292018?tool=bestpractice.com
[104]Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy: a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009 Jul;145(1):14-21.
http://www.ncbi.nlm.nih.gov/pubmed/19398260?tool=bestpractice.com
Excessive uterine bleeding not responding to conservative treatments
Infertility with distortion of the endometrial cavity or tubal obstruction
Recurrent pregnancy loss with distortion of the endometrial cavity
Pain or pressure symptoms that interfere with quality of life
Urinary tract symptoms (frequency and/or symptoms of obstruction)
Iron deficiency anaemia secondary to chronic blood loss.
The route of myomectomy - abdominal, laparoscopic, robotic, or hysteroscopic - largely depends on the size, location, and number of fibroids. Risk factors for recurrence of uterine fibroids include age 30 to 40 years and the presence of multiple fibroids; the more fibroids at the time of surgery, the higher the risk of recurrence. The 5-year re-intervention rates for abdominal, laparoscopic, and hysteroscopic myomectomies compared with other uterine preservation treatment options are 17%, 20%, and 28%, respectively.[105]Davis MR, Soliman AM, Castelli-Haley J, et al. Reintervention Rates After Myomectomy, Endometrial Ablation, and Uterine Artery Embolization for Patients with Uterine Fibroids. J Womens Health (Larchmt). 2018 Oct;27(10):1204-1214.
https://www.doi.org/10.1089/jwh.2017.6752
http://www.ncbi.nlm.nih.gov/pubmed/30085898?tool=bestpractice.com
It is important to recognise that fibroid recurrence does not indicate symptom recurrence or the need for re-intervention. Significant haemorrhage as a complication of myomectomy can result in emergent hysterectomy in a small number of cases.[106]Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008 Jan;100(1):4-9.
http://www.ncbi.nlm.nih.gov/pubmed/17894936?tool=bestpractice.com
[107]Iverson RE Jr, Chelmow D, Strohbehn K, et al. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996 Sep;88(3):415-9.
http://www.ncbi.nlm.nih.gov/pubmed/8752251?tool=bestpractice.com
Myomectomy by laparotomy: abdominal myomectomy by laparotomy is associated with longer surgical time and greater blood loss compared with hysterectomy and is associated with a 15% recurrence rate for uterine fibroids. Ten percent of women undergoing myomectomy through an abdominal incision will subsequently undergo hysterectomy within 5 to 10 years for persistent symptoms.[108]Lefebvre G, VIlos G, Allaire C, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2003 May;25(5):396-418.
http://www.ncbi.nlm.nih.gov/pubmed/12738981?tool=bestpractice.com
The usual surgical risks apply, including bleeding, infection, contiguous organ injury, and unintended hysterectomy. Cases of uterine rupture in pregnancies subsequent to myomectomy have been reported in the literature. Some authorities recommend managing patients with history of a myomectomy as you would for vaginal birth after caesarean candidates. Patients should be counselled regarding this low risk of uterine rupture in subsequent pregnancies following myomectomy, regardless of route of removal.
Myomectomy by laparoscopy: the open abdominal approach was the most common route used for myomectomy. However, with the advent of minimally invasive surgical techniques, laparoscopic myomectomy became more widely performed and accepted by many as the 'gold standard' approach to myomectomy.[109]Herrmann A, De Wilde R L. Laparoscopic myomectomy—the gold standard. Gynecol Minim Invasive Ther. 2014 May;3(2):31-8.
https://www.sciencedirect.com/science/article/pii/S2213307014000069
This minimally invasive surgery is completed through a number of small abdominal incisions. Careful patient selection based on size, location, and number of fibroids is important in deciding whether the patient is a candidate for laparoscopic myomectomy. If the mass is between 5 to 7 centimetres in diameter, the laparoscopic approach may be more appropriate and recovery is more rapid than with the abdominal approach. However, risk of recurrence is higher, particularly in cases with multiple fibroids.[110]Ming X, Ran XT, Li N, et al. Risk of recurrence of uterine leiomyomas following laparoscopic myomectomy compared with open myomectomy. Arch Gynecol Obstet. 2020 Jan;301(1):235-42.
http://www.ncbi.nlm.nih.gov/pubmed/31781891?tool=bestpractice.com
[111]Kotani Y, Tobiume T, Fujishima R, et al. Recurrence of uterine myoma after myomectomy: open myomectomy versus laparoscopic myomectomy. J Obstet Gynaecol Res. 2018 Feb;44(2):298-302.
https://obgyn.onlinelibrary.wiley.com/doi/full/10.1111/jog.13519
http://www.ncbi.nlm.nih.gov/pubmed/29227004?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Laparoscopy shows the presence of a large right posterolateral subserosal fibroid; note the close proximity of this uterine fibroid to the right ovary (potential for misdiagnosis as an adnexal mass)From the personal collection of Dr M.F. Mitwally and Dr R.J. Fischer; used with permission [Citation ends].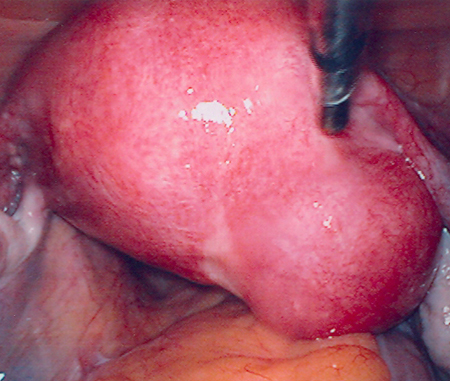
Robotic myomectomy: this technique was developed to overcome the difficulties of laparoscopic myomectomy and broaden the patient pool candidacy for the minimally invasive surgical approach.[112]Laveaux SM, Advincula AP. Robot-assisted laparoscopic myomectomy (RALM). In: El-Ghobashy A, Ind T, Persson J, et al, eds. Textbook of gynecologic robotic surgery. Cham: Springer; 2018. Laparoscopic myomectomy can be performed with robotic assistance. Multiple studies have shown the safety, efficacy, and feasibility of robotic myomectomy.[113]Lonnerfors C. Robot-assisted myomectomy. Best Pract Res Clin Obstet Gynaecol. 2018 Jan;46:113-119.
https://www.doi.org/10.1016/j.bpobgyn.2017.09.005
http://www.ncbi.nlm.nih.gov/pubmed/29103894?tool=bestpractice.com
[114]Iavazzo C, Mamais I, Gkegkes ID. Robotic assisted vs laparoscopic and/or open myomectomy: systematic review and meta-analysis of the clinical evidence. Arch Gynecol Obstet. 2016 Jul;294(1):5-17.
http://www.ncbi.nlm.nih.gov/pubmed/26969650?tool=bestpractice.com
Both robotic myomectomy and laparoscopic myomectomy are minimally invasive surgical options and associated with less blood loss, shorter hospital stay, quicker recovery times, and fewer complications and higher costs than laparotomy.[114]Iavazzo C, Mamais I, Gkegkes ID. Robotic assisted vs laparoscopic and/or open myomectomy: systematic review and meta-analysis of the clinical evidence. Arch Gynecol Obstet. 2016 Jul;294(1):5-17.
http://www.ncbi.nlm.nih.gov/pubmed/26969650?tool=bestpractice.com
[115]Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality Rates in Benign Laparoscopic and Robotic Gynecologic Surgery: A Systematic Review and Meta-analysis. J Minim Invasive Gynecol. 2020 Mar - Apr;27(3):603-612.e1.
https://www.doi.org/10.1016/j.jmig.2019.10.005
http://www.ncbi.nlm.nih.gov/pubmed/31627007?tool=bestpractice.com
[116]Sheu BC, Huang KJ, Huang SC, et al. Comparison of uterine scarring between robot-assisted laparoscopic myomectomy and conventional laparoscopic myomectomy. J Obstet Gynaecol. 2020 Oct;40(7):974-980.
https://www.doi.org/10.1080/01443615.2019.1678015
http://www.ncbi.nlm.nih.gov/pubmed/31790613?tool=bestpractice.com
The benefits of both robotic myomectomy and laparoscopic myomectomy compared with open abdominal myomectomy are well documented. Furthermore, there is no significant difference in patient outcomes compared with laparoscopic myomectomy. Candidate selection for robotic myomectomy is geared towards ensuring a successful procedure and minimising the risk of conversion to other surgical procedures.[117]Unger CA, Lachiewicz MP, Ridgeway B. Risk factors for robotic gynecologic procedures requiring conversion to other surgical procedures. Int J Gynaecol Obstet. 2016 Dec;135(3):299-303.
https://www.doi.org/10.1016/j.ijgo.2016.06.016
http://www.ncbi.nlm.nih.gov/pubmed/27591050?tool=bestpractice.com
[118]Dubuisson JB, Fauconnier A, Fourchotte V, et al. Laparoscopic myomectomy: predicting the risk of conversion to an open procedure. Hum Reprod. 2001 Aug;16(8):1726-31.
https://www.doi.org/10.1093/humrep/16.8.1726
http://www.ncbi.nlm.nih.gov/pubmed/11473973?tool=bestpractice.com
[119]American College of Obstetricians and Gynecologists. Committee opinion no. 810: robot-assisted surgery for noncancerous gynecologic conditions. Sep 2020 [internet publication].
https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/09/robot-assisted-surgery-for-noncancerous-gynecologic-conditions
For both laparoscopic and robotic myomectomy, fibroid location, size, and number, the patient's body habitus, and the relative size of the uterus to the length of the patient's torso are factors that must be considered. A preoperative MRI is highly recommended for fibroid mapping and to exclude the presence of adenomyosis. Robotic myomectomy is usually not offered to patients with fibroids >15 cm, a single fibroid >15 cm, or if the uterus is <5 fingerbreadths above the umbilicus.[112]Laveaux SM, Advincula AP. Robot-assisted laparoscopic myomectomy (RALM). In: El-Ghobashy A, Ind T, Persson J, et al, eds. Textbook of gynecologic robotic surgery. Cham: Springer; 2018.
Myomectomy by hysteroscopy: hysteroscopic myomectomy is a minimally invasive procedure that is the first-line treatment for intracavity International Federation of Gynecology and Obstetrics (FIGO) type 0, 1, and 2 fibroids. Preoperative sonogram and/or sonohysterography is mandatory to determine the location of the fibroid in relation to the uterine wall and the extent of protrusion into the uterine cavity.[120]Fernandez H, Sefrioui O, Virelizier C, et al. Hysteroscopic resection of submucosal myomas in patients with infertility. Hum Reprod. 2001 Jul;16(7):1489-92.
http://www.ncbi.nlm.nih.gov/pubmed/11425835?tool=bestpractice.com
[121]Bajekal N, Li TC. Fibroids, infertility and pregnancy wastage. Hum Reprod Update. 2000 Nov-Dec;6(6):614-20.
http://www.ncbi.nlm.nih.gov/pubmed/11129696?tool=bestpractice.com
[122]Di Spiezio Sardo A, Mazzon I, Bramante S, et al. Hysteroscopic myomectomy: a comprehensive review of surgical techniques. Hum Reprod Update. 2008 Mar-Apr;14(2):101-19.
https://academic.oup.com/humupd/article/14/2/101/609051
http://www.ncbi.nlm.nih.gov/pubmed/18063608?tool=bestpractice.com
Successful hysteroscopic myomectomy must consider the size, number, and location of fibroids. Furthermore, with type 2 lesions, the relationship of the deepest aspect of the fibroid(s) to the uterine serosa must be known. Guidelines recommend that fibroids up to 4-5 cm be removed by experienced surgeons to improve the chance of successful resection.[123]American Association of Gynecologic Laparoscopists (AAGL): Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012 Mar-Apr;19(2):152-71.
https://www.doi.org/10.1016/j.jmig.2011.09.005
http://www.ncbi.nlm.nih.gov/pubmed/22381967?tool=bestpractice.com
It is important to note that type 2 fibroids are most likely to require multistage procedures compared with type 0 and 1 fibroids. If an intracavitary fibroid traverses the myometrium and extends to the uterine serosa, hysteroscopic myomectomy is considered unsafe and should not be performed.
Regardless of the technique used for hysteroscopic myomectomy (resectoscope or morcellator), there is evidence to suggest that the risk of fluid overload is directly related to the duration of the procedure, the diameter of the leiomyoma(s), and the proportion of the fibroid that is in the myometrium.[124]Emanuel MH, Hart A, Wamsteker K, et al. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997 Nov;68(5):881-6.
https://www.doi.org/10.1016/s0015-0282(97)00335-x
http://www.ncbi.nlm.nih.gov/pubmed/9389820?tool=bestpractice.com
Additional significant but rare risks of hysteroscopic myomectomy include uterine perforation, electrical burns to genital organs and bowel, as well as heavy bleeding, which may require emergent hysterectomy.[125]Royal College of Obstetricians and Gynaecologists (RCOG); British Society for Gynaecological Endoscopy. Best practice in outpatient hysteroscopy. Green-top guideline no. 59. Mar 2011 [internet publication].
https://www.rcog.org.uk/globalassets/documents/guidelines/gtg59hysteroscopy.pdf
If hysteroscopy is not feasible, either an abdominal, laparoscopic, or robotic approach can be considered.
Uterine artery embolisation
Patients with significant obesity, diabetes, or hypertension, and those with serious cardiac or pulmonary dysfunction represent a high-risk group for major surgery and may not be candidates for hysterectomy.[48]American Congress of Obstetricians and Gynecologists. ACOG practice bulletin number 65: management of endometrial cancer. Obstet Gynecol. 2006 Apr;107(4):952.
http://www.ncbi.nlm.nih.gov/pubmed/16582139?tool=bestpractice.com
Uterine artery embolisation (UAE) has been shown to provide good short-term relief of bulk-related symptoms and reduction in heavy menstrual bleeding associated with uterine fibroids. Its impact on future fertility and future pregnancy outcome as well as long-term efficacy is currently unknown and therefore desire for future fertility is a relative contraindication to this procedure.[126]American Congress of Obstetricians and Gynecologists. ACOG committee opinion: uterine artery embolization. Obstet Gynecol. 2004 Feb;103(2):403-4.
http://www.ncbi.nlm.nih.gov/pubmed/14754718?tool=bestpractice.com
In treating fibroid-related symptoms, it is similar to the response rate seen following myomectomy (87.5% for UAE versus 93.3% for myomectomy). There is, however, a greater likelihood of follow-up surgery following UAE for persistent symptomatic fibroids.
UAE should be considered only in women not desiring any future fertility. However, pregnancies have been known to occur following UAE, with significant adverse obstetric outcomes, in particular significantly higher miscarriage rates, as well as other adverse events, including postpartum haemorrhage and increased risk for caesarean delivery.[127]Homer H, Saridogan E. Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil Steril. 2010 Jun;94(1):324-30.
http://www.ncbi.nlm.nih.gov/pubmed/19361799?tool=bestpractice.com
[128]Carranza-Mamane B, Havelock J, Hemmings R, et al; Society of Obstetricians and Gynaecologists of Canada. The management of uterine fibroids in women with otherwise unexplained infertility. J Obstet Gynaecol Can. 2015 Mar;37(3):277-85.
https://www.jogc.com/article/S1701-2163(15)30318-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26001875?tool=bestpractice.com
Therefore, it is important to ensure that women are aware of this and offered contraception if required.
Compared with hysterectomy and myomectomy, UAE is associated with a reduction in length of hospital stay and faster return to normal daily activities. Furthermore, one Cochrane review found that patient satisfaction with outcome (at 2 years of follow-up) is similar for UAE, hysterectomy, and myomectomy. Although there were more minor complications (vaginal discharge, postpuncture haematoma, and postembolisation syndrome) related to UAE, there were no significant differences in serious/long-term complications between myomectomy and UAE. However, there was an increased likelihood of requiring further surgical intervention within 2 to 5 years of UAE, and this may balance out any initial cost advantage of UAE.[129]Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014 Dec 26;(12):CD005073.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD005073.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/25541260?tool=bestpractice.com
[  ]
Is there RCT evidence to support the use of uterine artery embolization (UAE) in women with symptomatic uterine fibroids?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2387/fullShow me the answer
]
Is there RCT evidence to support the use of uterine artery embolization (UAE) in women with symptomatic uterine fibroids?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2387/fullShow me the answer
Long-term studies of UAE are lacking; however, in 1 study comparing outcomes of UAE and abdominal myomectomy, the former resulted in a 29% rate of further invasive therapy during the 3 to 5 years of follow-up as compared with 3% in the myomectomy group.[73]Broder MS, Goodwin S, Chen G, et al. Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002 Nov;100(5 Pt 1):864-8.
http://www.ncbi.nlm.nih.gov/pubmed/12423842?tool=bestpractice.com
Risks of this procedure include bleeding, infection, allergic reactions to iodinated contrast dye, femoral artery puncture site haematomas, incomplete uterine artery occlusion, and inadvertent embolisation of other organs. Most complications of UAE tend to occur more than 30 days after the procedure. Late complications include vaginal discharge, expulsion of fibroid material, and infection.[130]Royal College of Obstetricians and Gynaecologists; Royal College of Radiologists. Clinical recommendations on the use of uterine artery embolisation (UAE) in the management of fibroids. Dec 2013 [internet publication].
https://www.rcog.org.uk/guidance/browse-all-guidance/other-guidelines-and-reports/uterine-artery-embolisation-in-the-management-of-fibroids
Also, there has been growing concern over the risk of unintended embolisation of the utero-ovarian circulation, leading to reduction of ovarian blood supply with subsequent impairment of ovarian reserve. However, one systematic review showed that UAE for uterine fibroids does not seem to affect ovarian reserve.[131]El Shamy T, Amer SAK, Mohamed AA, et al. The impact of uterine artery embolization on ovarian reserve: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020 Jan;99(1):16-23.
https://www.doi.org/10.1111/aogs.13698
http://www.ncbi.nlm.nih.gov/pubmed/31370100?tool=bestpractice.com
Hysterectomy where fertility is no longer required
Hysterectomy is the definitive and most common treatment for symptomatic uterine fibroids, primarily because it provides an absolute cure with no possibility of recurrence.[9]American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 228: management of symptomatic uterine leiomyomas. Jun 2021 [internet publication].
https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
It is indicated in women who do not wish to preserve fertility, who are good surgical candidates, and who have been adequately counselled regarding risks and alternatives; hysterectomy is associated with a high level of satisfaction and efficacy.[108]Lefebvre G, VIlos G, Allaire C, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2003 May;25(5):396-418.
http://www.ncbi.nlm.nih.gov/pubmed/12738981?tool=bestpractice.com
There is no evidence that hysterectomy interferes with sexuality or coital orgasm. Aside from the usual surgical risks, hysterectomy for uterine fibroids carries a relatively small risk of damage to the urinary tract, bowel, and vagina as well as later development of vaginal vault prolapse.[72]Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004 Aug;104(2):393-406.
http://www.ncbi.nlm.nih.gov/pubmed/15292018?tool=bestpractice.com
There are four generally recognised surgical approaches to hysterectomy for benign conditions of the uterus such as leiomyomata uteri.
Abdominal hysterectomy: the abdominal approach involves a relatively large incision through the abdominal wall.
Vaginal hysterectomy: this is accomplished solely through an incision in the upper vagina.
Laparoscopic hysterectomy: this approach either facilitates delivery of the uterus through a vaginal incision (laparoscopic-assisted vaginal hysterectomy) or is completely accomplished through a number of small (approximately 1 cm) incisions in the abdomen (total laparoscopic hysterectomy).
Robotic hysterectomy: similar to vaginal and laparoscopic hysterectomy, this approach is a minimally invasive option for hysterectomy. Robotic hysterectomy is completely accomplished through a number of small incisions as in total laparoscopic hysterectomy.
Choice of approach is dependent on a number of factors including uterine size and mobility, the surgeon's experience and expertise, and the patient's medical status and preference. Numerous advantages of vaginal hysterectomy over abdominal hysterectomy have been identified, including shorter duration of hospital stay, speedier return to normal activities, and fewer unspecified infections or febrile episodes. Similar advantages have been noted for laparoscopic hysterectomy; however, this approach is associated with longer operating time and more bladder and ureteral injuries compared with abdominal hysterectomy. The use of robotically assisted hysterectomy for benign gynaecological conditions has been increasing. However, the role of robotics for benign hysterectomy has not been clearly delineated.[119]American College of Obstetricians and Gynecologists. Committee opinion no. 810: robot-assisted surgery for noncancerous gynecologic conditions. Sep 2020 [internet publication].
https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/09/robot-assisted-surgery-for-noncancerous-gynecologic-conditions
In 2015, the American College of Obstetricians and Gynecologists (ACOG) reaffirmed a 2009 statement endorsing the vaginal approach as the preferred route for benign hysterectomy because of its lower complication rates and well-documented advantages.[132]American College of Obstetricians and Gynecologists. Committee opinion no. 628: robotic surgery in gynecology. Obstet Gynecol. 2015 Mar;125(3):760-7.
http://www.ncbi.nlm.nih.gov/pubmed/25730256?tool=bestpractice.com
[133]American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009 Nov;114(5):1156-1158.
https://www.doi.org/10.1097/AOG.0b013e3181c33c72
http://www.ncbi.nlm.nih.gov/pubmed/20168127?tool=bestpractice.com
ACOG and the American Association of Gynecologic Laparoscopists recommend a laparoscopic approach as an alternative approach when a vaginal route is not feasible.[134]AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011 Jan-Feb;18(1):1-3.
https://www.doi.org/10.1016/j.jmig.2010.10.001
http://www.ncbi.nlm.nih.gov/pubmed/21059487?tool=bestpractice.com
The primary goal of these recommendations is to avoid the morbidity of laparotomy whenever feasible.
[  ]
In women with benign gynecological disease, how do different approaches to hysterectomy compare at improving outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1225/fullShow me the answer
]
In women with benign gynecological disease, how do different approaches to hysterectomy compare at improving outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1225/fullShow me the answer
The lack of clarity with regards to robotics is due to the dearth of evidence in the literature to prove its role for hysterectomy over laparoscopic and vaginal routes. A 2019 Cochrane review addressing robotic surgery in gynaecology concluded, with regards to benign hysterectomy, that the effectiveness and safety of robotics compared with laparoscopy is of low certainty but that surgical complication rates between both routes are comparable.[135]Lawrie TA, Liu H, Lu D, et al. Robot-assisted surgery in gynaecology. Cochrane Database Syst Rev. 2019 Apr 15;4:CD011422.
https://www.doi.org/10.1002/14651858.CD011422.pub2
http://www.ncbi.nlm.nih.gov/pubmed/30985921?tool=bestpractice.com
Increased surgical time and cost of robotic surgery are two points of major controversy and debate. Regarding surgical time, studies comparing the robotic versus laparoscopic approach generally show equivalent or longer surgical time with the robotic approach and otherwise no clinical or statistically significant differences between both approaches.[136]Martínez-Maestre MA, Gambadauro P, González-Cejudo C, et al. Total laparoscopic hysterectomy with and without robotic assistance: a prospective controlled study. Surg Innov. 2014 Jun;21(3):250-5.
https://www.doi.org/10.1177/1553350613492023
http://www.ncbi.nlm.nih.gov/pubmed/23833240?tool=bestpractice.com
[137]Paraiso MF, Ridgeway B, Park AJ, et al. A randomized trial comparing conventional and robotically assisted total laparoscopic hysterectomy. Am J Obstet Gynecol. 2013 May;208(5):368.e1-7.
https://www.doi.org/10.1016/j.ajog.2013.02.008
http://www.ncbi.nlm.nih.gov/pubmed/23395927?tool=bestpractice.com
[138]Sarlos D, Kots L, Stevanovic N, et al. Robotic compared with conventional laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2012 Sep;120(3):604-11.
https://www.doi.org/10.1097/AOG.0b013e318265b61a
http://www.ncbi.nlm.nih.gov/pubmed/22914470?tool=bestpractice.com
Studies comparing costs of minimally invasive surgical hysterectomy - robotic versus laparoscopic versus vaginal - show mixed results.[139]Swenson CW, Kamdar NS, Harris JA, et al. Comparison of robotic and other minimally invasive routes of hysterectomy for benign indications. Am J Obstet Gynecol. 2016 Nov;215(5):650.e1-650.e8.
https://www.doi.org/10.1016/j.ajog.2016.06.027
http://www.ncbi.nlm.nih.gov/pubmed/27343568?tool=bestpractice.com
[140]Lönnerfors C, Reynisson P, Persson J. A randomized trial comparing vaginal and laparoscopic hysterectomy vs robot-assisted hysterectomy. J Minim Invasive Gynecol. 2015 Jan;22(1):78-86.
https://www.doi.org/10.1016/j.jmig.2014.07.010
http://www.ncbi.nlm.nih.gov/pubmed/25045857?tool=bestpractice.com
In an effort to address these cost concerns, strategies are being implemented by robotic surgeons to maximise the cost-effectiveness of robotic surgery, including minimising the number of ports and robotic instruments used per case, training dedicated robotic teams to improve work-flow efficiency, and reducing surgical time.[141]Bogliolo S, Ferrero S, Cassani C, et al. Single-site Versus Multiport Robotic Hysterectomy in Benign Gynecologic Diseases: A Retrospective Evaluation of Surgical Outcomes and Cost Analysis. J Minim Invasive Gynecol. 2016 May-Jun;23(4):603-9.
https://www.doi.org/10.1016/j.jmig.2016.02.006
http://www.ncbi.nlm.nih.gov/pubmed/26898895?tool=bestpractice.com
 ]
It can cause transient elevations in transaminases.[91][92]
]
It can cause transient elevations in transaminases.[91][92]
 ]
]
 ]
]