Aetiology
The exact aetiology of uterine fibroids is not completely understood. There is good evidence that uterine fibroids grow from a single mutated uterine smooth muscle cell and are thereby monoclonal tumours.[1][10] Initiation and promotion of abnormal growth of this single myometrial cell, however, is less well understood.[1] Chromosomal rearrangements including specific translocations have been identified in some specimens, which may be responsible for the initiation and proliferation of uterine fibroids.[11][12] Multiple leiomyomas develop de novo rather than through a metastatic mechanism.
Oestrogen and progesterone have been implicated in the promotion of monoclonal myometrial cell proliferation and growth of uterine fibroids.[1] Uterine fibroid proliferation may be the consequence of complex interactions between oestrogen, progesterone, and local growth factors, with oestrogen and progesterone equally responsible for the promotion of uterine fibroid growth.[13] Uterine fibroids have been shown to express gonadotrophin-releasing hormone (GnRH) receptors; administration of GnRH agonists has an antiproliferative effect.[14]
The most accepted current model for the origin of uterine fibroids is that it starts from a perturbed myometrial stem cell, probably because of early live environmental adverse exposures or related events, which acquire a MED12 mutation and become a fibroid tumour-forming stem cell. MED12 mutations from fibroid stem cells are very common and affect up to 70% of human fibroid lesions.[1][15] It seems that stem cells of fibroids have an important role in pathogenesis. Also the effect of the Wnt/beta-catenin pathway in paracrine signalling in stem cells of fibroids has been demonstrated.[16][17][18][19][20]
Pathophysiology
Studies have revealed the role of the extracellular matrix, transforming growth factor beta, and collagen structure in leiomyoma formation, providing evidence for molecular similarities between leiomyomas and keloids. Additionally, a model of development was created based on an abnormal response to tissue repair, disordered healing, and formation of an altered extracellular matrix.[21]
Uterine fibroids arise from the myometrial layer of the uterine corpus or, less commonly, the uterine cervix, and may occur singly or multiply. Fibroids may remain within the muscular layer (intramural) or protrude outwardly to become subserosal in location or inwardly towards the endometrial cavity, where they become known as submucous fibroids. Subserosal and submucosal fibroids may become pedunculated. Abnormal vaginal bleeding that often accompanies the presence of fibroids is felt to occur as a result of distortion of the endometrial lining and therefore is seen much more commonly with submucous fibroids. The histological changes in the endometrium and endometrial vasculature of the fibroid area, which are likely to be secondary to secreted humoral factors from fibroid tumours, lead to abnormal uterine bleeding.[22] For the same reason, cavity distortion can cause recurrent second trimester loss. Uterine fibroids that obstruct menstrual flow can cause dysmenorrhoea. Large uterine fibroids, regardless of location, can cause mass effects on contiguous organs such as the bowel and bladder and cause symptoms of urinary frequency, urgency, and incontinence as well as constipation. They can outstrip their blood supply and cause acute or chronic pain as they degenerate. Pedunculated submucous uterine fibroids can dilate the uterine cervix and prolapse into the vagina where they can become infected.
Various mechanisms have been proposed to explain the strong association between heavy menses and uterine fibroids. They have included ulceration over the surface of submucous uterine fibroids, anovulation associated with uterine fibroids, increased endometrial surface area, and interference with normal uterine contractility. To date, none of these explanations have been conclusively validated by clinical research.[23]
More recently, research into this area has centred on vascular dysregulation, thought to be mediated by a number of growth factors. It is now hypothesised that fibroid-associated bleeding is related to dilatation of the small veins (venules) within the myometrium and endometrium of uteri containing fibroids, thus interfering with the haemostatic actions of platelets and fibrin plugs.[24] Nevertheless, a cause and effect has not been established.
Classification
Anatomical classification[3][4]
1. Uterine body
Subserosal myoma: located just beneath the uterine serosa and generally growth occurs outward into the pelvic cavity; they may become pedunculated.
Intramural myoma: located within the muscular layer of the uterus.
Submucosal myoma: located just beneath the endometrial lining and generally growth occurs into the uterine cavity; they may become pedunculated.
2. Cervical: located within the cervix.
3. Broad ligament: located between the two layers of the broad ligament.
Many fibroids have more than one localisation in the uterus compartments. The exact position of the fibroids may change the treatment options. The International Federation of Gynecology and Obstetrics (FIGO) classification system for fibroid location can help evaluate the less invasive options.[Figure caption and citation for the preceding image starts]: FIGO leiomyoma subclassification systemMunro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018 Dec;143(3):393-408; used with permission. [Citation ends].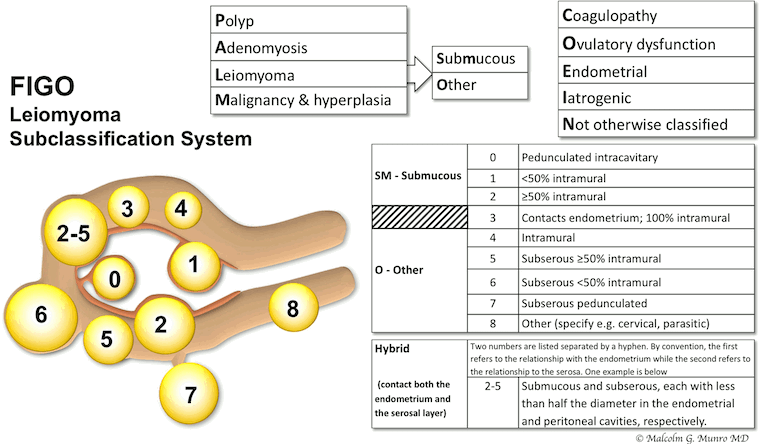
Use of this content is subject to our disclaimer