Treatment for patients with Brugada syndrome (BrS) consists of conservative management and primary and secondary prevention of serious arrhythmic events.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
This aims to prevent serious arrhythmic events, while avoiding potential complications of any treatments.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Potential treatments include implantable cardioverter defibrillator (ICD), pharmacological therapy, and radiofrequency catheter ablation.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Note that the distinction between confirmed and probable BrS is not significant in determining the management; this is guided by the patient’s presentation, particularly whether they are symptomatic or asymptomatic. Most patients who are asymptomatic require conservative management only.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Management summary for Brugada syndromeAdapted from Krahn AD et al. J Am Coll Cardiol EP. 2022 Mar, 8 (3) 386-405; used with permission [Citation ends].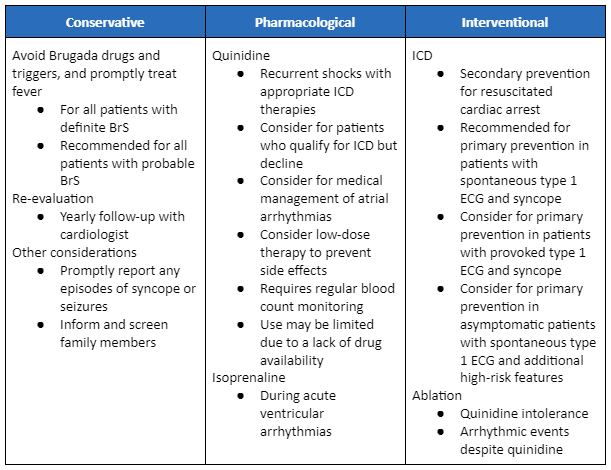
Acute ventricular arrhythmia
Intravenous isoprenaline should be given during an acute ventricular arrhythmia, including electrical storm (>2 episodes of ventricular tachycardia or ventricular fibrillation in 24 hours) if the mechanism is due to short-coupled premature ventricular complex-induced ventricular fibrillation.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
US guidelines recommend also considering quinidine for patients who experience electrical storm.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
[81]Eifling M, Razavi M, Massumi A. The evaluation and management of electrical storm. Tex Heart Inst J. 2011;38(2):111-21.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3066819
http://www.ncbi.nlm.nih.gov/pubmed/21494516?tool=bestpractice.com
In practice, this is given once they have been stabilised with isoprenaline.
Difficulty in obtaining quinidine can limit its use; phosphodiesterase-III inhibitors (cilostazol or milrinone) may be considered as an alternative, although supportive data are limited.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Risk stratification
Risk stratify all patients to identify those who are at increased risk of serious arrhythmic events.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Risk stratification can aid decision making for the management of Brugada syndrome, particularly when considering an ICD for primary prevention of serious arrhythmic events.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[72]Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. J Arrhythm. 2020 Jun 15;36(4):553-607.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/32782627
http://www.ncbi.nlm.nih.gov/pubmed/32782627?tool=bestpractice.com
There are currently no risk stratification tools that have proved effective in clinical practice.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
The Sieira risk score has been proposed for predicting sudden death in patients with BrS, but has not been externally validated.[57]Honarbakhsh S, Providencia R, Garcia-Hernandez J, et al. A Primary prevention clinical risk score model for patients with Brugada syndrome (BRUGADA-RISK). JACC Clin Electrophysiol. 2021 Feb;7(2):210-2.
https://www.sciencedirect.com/science/article/pii/S2405500X20308197?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/33602402?tool=bestpractice.com
[82]Sieira J, Conte G, Ciconte G, et al. A score model to predict risk of events in patients with Brugada Syndrome. Eur Heart J. 2017 Jun 7;38(22):1756-63.
https://academic.oup.com/eurheartj/article/38/22/1756/3098019?login=false
http://www.ncbi.nlm.nih.gov/pubmed/28379344?tool=bestpractice.com
[83]Chow JJ, Leong KMW, Yazdani M, et al. A multicenter external validation of a score model to predict risk of events in patients with Brugada syndrome. Am J Cardiol. 2021 Dec 1;160:53-9.
http://www.ncbi.nlm.nih.gov/pubmed/34610873?tool=bestpractice.com
[84]Probst V, Goronflot T, Anys S, et al. Robustness and relevance of predictive score in sudden cardiac death for patients with Brugada syndrome. Eur Heart J. 2021 May 1;42(17):1687-95.
https://academic.oup.com/eurheartj/article/42/17/1687/6027696?login=false
http://www.ncbi.nlm.nih.gov/pubmed/33289793?tool=bestpractice.com
However, established features that are associated with the greatest risk of serious arrhythmic events are:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[85]Gonzalez Corcia MC, Sieira J, Pappaert G, et al P. A clinical score model to predict lethal events in young patients (≤19 years) with the Brugada syndrome. Am J Cardiol. 2017 Sep 1;120(5):797-802.
http://www.ncbi.nlm.nih.gov/pubmed/28728742?tool=bestpractice.com
[86]Andorin A, Behr ER, Denjoy I, et al. Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm. 2016 Jun;13(6):1274-82.
http://www.ncbi.nlm.nih.gov/pubmed/26921764?tool=bestpractice.com
[87]Priori SG, Gasparini M, Napolitano C, et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59(1):37-45.
https://www.sciencedirect.com/science/article/pii/S073510971104530X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/22192666?tool=bestpractice.com
[88]Priori SG, Napolitano C, Gasparini M, et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105(11):1342-7.
https://www.ahajournals.org/doi/10.1161/hc1102.105288?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/11901046?tool=bestpractice.com
Resuscitated cardiac arrest
History of cardiogenic syncope
Spontaneous type 1 Brugada pattern on ECG.
Other features that are associated with increased risk of serious arrhythmic events are:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Sudden cardiac death in a young (<35 years) first-degree relative
Greater ‘Brugada burden’ on ECG, which includes:
Other ECG markers such as early repolarisation pattern and QRS fragmentation.[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[72]Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: use the right tool for the right outcome, in the right population. J Arrhythm. 2020 Jun 15;36(4):553-607.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/32782627
http://www.ncbi.nlm.nih.gov/pubmed/32782627?tool=bestpractice.com
[89]Conte G, de Asmundis C, Sieira J, et al. Prevalence and clinical impact of early repolarization pattern and QRS-fragmentation in high-risk patients with Brugada syndrome. Circ J. 2016 Sep 23;80(10):2109-16.
http://www.ncbi.nlm.nih.gov/pubmed/27558008?tool=bestpractice.com
Age and sex have limited effect on risk of serious arrhythmic events, although age ≥55 at diagnosis is associated with a more benign prognosis, with no increased mortality compared with the general population.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Consider an electrophysiological study with programmed ventricular stimulation using up to two extrastimuli for further risk stratification in some patients, although this remains controversial.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
It should not be used routinely because it is invasive and puts the patient at risk of complications, as well as having issues around reproducibility of results.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
One multi-centre pooled analysis showed that in patients with BrS, arrhythmias induced with electrophysiological studies were associated with a higher future risk of ventricular arrhythmia.[90]Sroubek J, Probst V, Mazzanti A, et al. Programmed ventricular stimulation for risk stratification in the Brugada syndrome: A pooled analysis. Circulation. 2016;133(7):622-30.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4758872
http://www.ncbi.nlm.nih.gov/pubmed/26797467?tool=bestpractice.com
However, it may be useful in certain circumstances (e.g., where the decision to use an implantable cardioverter defibrillator for primary prevention is unequivocal).[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
Conservative management
Use conservative management for all patients with definite/probable or suspected BrS, although some patients may need other additional treatments alongside this.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Asymptomatic patients with inducible (particularly drug-induced) Brugada pattern on ECG usually require conservative management only.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
Conservative management includes:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Avoidance of drugs that could exacerbate the Brugada pattern (for further information see:
BrugadaDrugs.org: safe drug use and the Brugada syndrome
Opens in new window).[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
These include:
Antiarrhythmics (particularly sodium-channel blockers)
Psychotropic drugs
Anaesthetics
Certain over-the-counter drugs (e.g., antihistamines)
Illicit drugs (e.g., cannabis, cocaine).
Prompt treatment of fever[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Avoidance of metabolic disturbance (e.g., hypokalaemia, hyperkalaemia, metabolic acidosis)
Avoidance of alcohol intoxication[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Implantable cardioverter defibrillator (ICD)
An ICD is recommended for all patients with BrS who have been resuscitated from cardiac arrest for secondary prevention of sudden cardiac death.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
[91]National Institute for Health and Care Excellence. Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure. Jun 2014 [internet publication].
https://www.nice.org.uk/guidance/ta314
However, the decision to use an ICD for primary prevention of sudden cardiac death due to BrS is less clear cut and needs to weigh up the risk of serious arrhythmic events with the risk of ICD-related complications.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
The patient’s life expectancy should also be taken into account; ICD may not be appropriate if meaningful survival is <1 year.[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
Regarding choice of ICD, a dual chamber system is recommended in most patients, particularly those prone to atrial arrhythmias (e.g., those carrying the SN5CA gene).[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
However, a subcutaneous device may be preferred in young patients who don’t require pacing, because this has a lower risk of intravascular infection.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Pharmacological therapy
Quinidine
Quinidine is a class 1a antiarrhythmic agent that has many effects.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
The most significant of these is inhibition of the cardiac transient outward potassium current, which prolongs the refractory period.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Quinidine is useful to suppress ventricular arrhythmias, and may also be used for medical management of atrial arrhythmias.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
It should be considered in patients who:[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
Have an ICD and experience recurrent shocks[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
Decline or are unsuitable for an ICD[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Experience electrical storm (>2 episodes of ventricular tachycardia or ventricular fibrillation in 24 hours).[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
See 'Acute ventricular arrhythmia' above
Are asymptomatic but have a spontaneous type I Brugada pattern on ECG[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Experience asymptomatic ventricular arrhythmia.[68]Arnar DO, Mairesse GH, Boriani G, et al. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). Europace. 2019 Mar 18;21(6):844–5.
https://academic.oup.com/europace/article/21/6/844/5382236?login=false
http://www.ncbi.nlm.nih.gov/pubmed/30882141?tool=bestpractice.com
Be aware that significant adverse effects and difficulty in obtaining the drug can limit its use.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
Monitor the patient's FBC for anaemia and thrombocytopenia.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Phosphodiesterase-III inhibitors
Phosphodiesterase-III inhibitors (cilostazol or milrinone) may be considered for patients with BrS (as an alternative to quinidine); however, supportive data are limited.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Radiofrequency catheter ablation
Radiofrequency catheter ablation is indicated in patients who:
Have recurrent ICD shocks, particularly if these are refractory to medical therapy[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Experience serious arrhythmic events despite optimised medical therapy[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
Are intolerant to, or would prefer not to have, medical therapy, or medical therapy is ineffective[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[5]Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019 Aug 1;21(8):1143-4.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7967791
http://www.ncbi.nlm.nih.gov/pubmed/31075787?tool=bestpractice.com
Have spontaneous type 1 Brugada pattern on ECG and symptomatic ventricular arrhythmias who are unsuitable for, or decline an ICD[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
Have a history of electrical storms.[51]Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013;10(12):e85-108.
https://core.ac.uk/reader/82269654?utm_source=linkout
http://www.ncbi.nlm.nih.gov/pubmed/23916535?tool=bestpractice.com
Significant contraindications to ablation include:[38]Nademanee K, Veerakul G, Chandanamattha P, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123(12):1270-9.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.110.972612?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/21403098?tool=bestpractice.com
Ventricular tachycardia or fibrillation caused by myocardial ischeamia, fever, or hypokalaemia
Presence of structural heart disease
Brain anoxic encephalopathy from cardiac arrests caused by ventricular tachycardia or fibrillation.
A combined endocardial and epicardial approach has been shown to modify triggers and substrate for ventricular arrhythmias.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
Genetic counselling
Arrange genetic counselling for all patients to facilitate screening of relatives.[1]Krahn AD, Behr ER, Hamilton R, et al. Brugada syndrome. JACC Clin Electrophysiol. 2022 Mar;8(3):386-405.
https://www.sciencedirect.com/science/article/pii/S2405500X2101080X?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35331438?tool=bestpractice.com
[7]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022 Oct 21;43(40):3997-4126.
https://academic.oup.com/eurheartj/article/43/40/3997/6675633?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
[18]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Oct;15(10):e190-e252.
https://www.heartrhythmjournal.com/article/S1547-5271(17)31249-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29097320?tool=bestpractice.com
[80]Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: A scientific statement from the American Heart Association. Circ Genom Precis Med. 2020 Aug;13(4):e000067.
https://www.ahajournals.org/doi/full/10.1161/HCG.0000000000000067?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/32698598?tool=bestpractice.com
See Screening.
