FDA approves olezarsen for the treatment of familial chylomicronemia syndrome
Olezarsen, a ligand conjugated antisense oligonucleotide targeting apo C-III, has been shown to reduce triglyceride (TG) levels by >50% and acute pancreatitis risk by >85% in patients with familial chylomicronemia syndrome (FCS).[71] Olezarsen has been approved by the US Food and Drug Administration as an adjunct to diet to reduce TG levels in adults with FCS. Note this indication is for a rare and severely affected subgroup of patients with hypertriglyceridaemia only. Olezarsen is not approved in Europe as yet.
Summary
Definition
History and exam
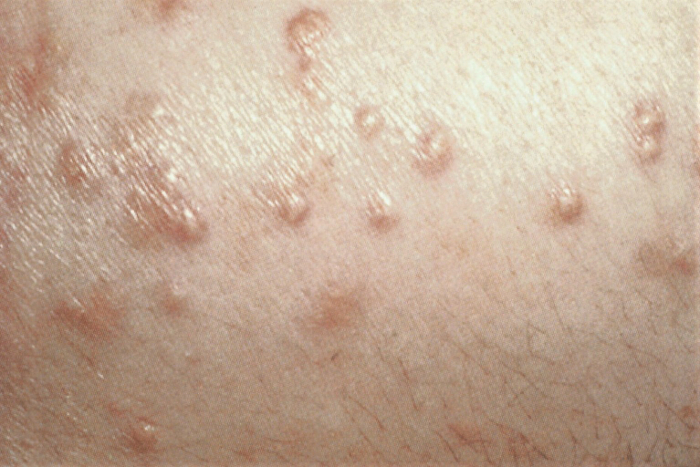
Other diagnostic factors
- increased BMI/waist circumference
- lipodystrophy
- features of coronary artery disease
- claudication
- neurological features
- recurrent abdominal pain
Risk factors
- family history of hyperlipidaemia
- high saturated fat diet
- high carbohydrate or high glycaemic index diet
- excessive alcohol consumption
- family or personal history of overweight/obesity
- family or personal history of diabetes
- insulin resistance
- liver disease
- renal disease
- HIV infection
- use of certain drugs
- Cushing syndrome
- inflammatory/immune disorders
- organ transplant
- hypothyroidism
- pregnancy
Diagnostic investigations
Investigations to consider
- apolipoprotein B
- fasting plasma glucose
- urea, creatinine
- urinary albumin/protein
- serum albumin
- thyroid-stimulating hormone
- liver function tests
- C-reactive protein
Treatment algorithm
Contributors
Authors
Robert A. Hegele, MD, FRCPC, Cert Endo, FACP, FAHA
Jacob J. Wolfe Distinguished Medical Research Chair
Martha Blackburn Chair in Cardiovascular Research
Distinguished University Professor of Medicine and Biochemistry
University of Western Ontario
London
Ontario
Canada
Disclosures
RAH reports consulting fees from Acasti, Aegerion, Akcea/Ionis, Amgen, Boston Heart, HLS Therapeutics, Novartis, Pfizer, Regeneron, Sanofi and UltraGenyx. RAH is an author of references cited in this topic.
Acknowledgements
Dr Robert A. Hegele would like to gratefully acknowledge Dr David Alexander Leaf, the previous contributor to this topic.
Disclosures
DAL declares that he has no competing interests.
Peer reviewers
Vinaya Simha, MBBS, MD
Associate Professor
Mayo Clinic
Rochester
MN
Disclosures
VS declares that he has no competing interests.
Use of this content is subject to our disclaimer