Approach
Clinicians trained in neonatal resuscitation, including tracheal intubation, should attend deliveries where there is meconium-stained amniotic fluid (MSAF).[53] Although some infants born through MSAF may have an uneventful course in the delivery room, and later others may experience mild to severe respiratory distress with full-blown MAS, paediatricians should properly assess the infant in the delivery room and develop appropriate management plans.
An initial approach is to provide general care and resuscitation as needed in the delivery room and to stabilise the infant.
Vigorous infant, no respiratory distress
Normal-term infants born through MSAF without a history of maternal group B streptococcal infection or other infections, who are vigorous at birth and manifest no respiratory distress, can be allowed to stay with the mother as a normal newborn after routine delivery room care.[53] Routine gastric aspiration in babies born through MSAF has no advantage in preventing MAS.[39] Treatment with antibiotics is not indicated in these babies.
Antibiotics are indicated in the presence of risk factors or laboratory findings suggestive of infection (e.g., chorioamnionitis, prolonged rupture of membranes, oligohydramnios, fetal heart rate abnormalities, post-maturity). Routine gastric aspiration in babies born through MSAF has no advantage in preventing MAS.[39] Broad-spectrum antibiotics used include ampicillin and gentamicin.[54] Treatment with antibiotics should be discontinued if 48-hour blood cultures are negative, unless there is clear evidence of site-specific infection.[54] If blood cultures are positive, antibiotics should be continued for up to 7 days.
Mild MAS
Infants with mild respiratory distress, tachypnoea, mild cyanosis, and retractions should be admitted to the neonatal intensive care unit (NICU) for treatment and observation.
Infants should be placed in an Isolette, or under an infant warmer, and oxygen saturation should be monitored continuously.
Oxygen should be given by hood or nasal cannula to maintain oxygen saturations of 92% to 97%. The infant usually may require an FiO₂ of <0.40 for a short duration of 48 to 72 hours. As the respiratory distress begins to improve, FiO₂ should be decreased by 5% at a time, as tolerated, depending on the pulse oximeter reading.
For nutritional support, intravenous fluids should be started on day 1. On subsequent days, switching to nasogastric or oral feeds, as tolerated, should be considered if the infant's respiratory status improves. If feedings are not adequate, intravenous fluid should be increased to meet the daily requirement.
Hypoglycaemia may be noted in babies with intrauterine growth restriction or severe hypoxic insult. Giving intravenous fluids containing glucose is indicated until hypoglycaemia resolves.
Antibiotics are indicated in the presence of risk factors or laboratory findings suggestive of infection (e.g., chorioamnionitis, prolonged rupture of membranes, oligohydramnios, fetal heart rate abnormalities, post-maturity). Broad-spectrum antibiotics used include ampicillin and gentamicin.[54] Treatment with antibiotics should be discontinued if 48-hour blood cultures are negative, unless there is clear evidence of site-specific infection.[54] If blood cultures are positive, antibiotics should be continued for up to 7 days.
Usually infants in this category recover in 3 to 5 days. However, if symptoms of moderate distress are present, despite the above management, the patient should be transferred to a level II or higher NICU for further care.
Moderate MAS
Patients in this group include infants with moderate respiratory distress who do not respond to the management described above or with moderate respiratory distress at presentation. They require continuous positive airway pressure (CPAP) or mechanical ventilation with high FiO₂, and are best managed at a level III NICU under the care of a neonatologist.
In infants with spontaneous breathing and good respiratory effort, CPAP should be started with nasal prongs if FiO₂ needs exceed 0.40 to maintain saturations within normal limits. CPAP should be avoided in the presence of air leaks and air trapping on chest x-ray (CXR). Complications include abdominal distension, air trapping because of underlying ball-valve mechanisms or excessive flow, and distending pressure. These potential complications warrant close monitoring. CPAP reduces the need for mechanical ventilation in infants with MAS who have peripheral oxygen saturations <90% and respiratory distress score >4.[55]
Treatment with intravenous broad-spectrum antibiotics (e.g., ampicillin and gentamicin) is initiated in all patients.
Supportive care includes parenteral nutrition with an amino acid solution and an intralipid solution later to meet the caloric requirements.
Infants who continue to exhibit respiratory distress despite the above management should be intubated and mechanically ventilated. Intubation criteria are FiO₂ >0.60, increased work of breathing or apnoea, and deteriorating ABG values showing low PaO₂ (<50 mmHg, PaCO₂ 70 mmHg, dropping pH to <7.25). Once the infant is intubated and stabilised, blood gases and CXR should be obtained to reassess the condition.
Pulmonary surfactant may be altered or inactivated in babies with MAS. A bolus dose of surfactant can be given as an additional treatment in this patient group, through the endotracheal tube immediately after intubation. Evidence suggests that surfactant administration in infants with moderate to severe MAS decreases the risk of progressive respiratory failure that requires support with extracorporeal membrane oxygenation (ECMO).[56][57][58] Pneumothorax should be treated with a chest tube before giving surfactant therapy. Lung lavage with diluted surfactant in intubated infants in small aliquots may be considered, especially in units where further ECMO support is not available. Lung lavage with diluted surfactant may be beneficial, but further clinical trials are needed to determine long-term outcomes.[59][60] [
 ]
Only an experienced specialist should perform lung lavage, as it is associated with severe desaturations.
]
Only an experienced specialist should perform lung lavage, as it is associated with severe desaturations.Infants with MAS are prone to developing hypotension, especially after receiving sedatives. Many infants are asphyxiated at birth and may have myocardial depression and hypotension. Inotropes such as dopamine, dobutamine, and adrenaline (epinephrine) to maintain a higher systemic pressure and avoid hypotension from simultaneous administration of sedatives. Due to dopamine’s non-selective systemic and pulmonary vasoconstriction it can often lead to increased pulmonary vascular resistance. Expert consensus therefore now supports the use of dobutamine and/or adrenaline to support cardiac function in MAS, particularly when pulmonary hypertension is suspected.[61][62]
Refractory patients should be transferred to a level IV NICU (if no ECMO available at level III) under the care of a neonatologist and with all the support systems to give inhaled nitric oxide (iNO) or ECMO.
Severe MAS
Patients in this group include infants who are refractory to mechanical ventilation with high oxygen inspiration and surfactant treatment or with severe respiratory distress at presentation. These infants are best managed at a level III NICU (or IV if no ECMO available at level III) under the care of a neonatologist.
Infants who are refractory to mechanical ventilation with high oxygen inspiration and surfactant treatment invariably have concomitant persistent pulmonary hypertension (PPHN). iNO should be given with a starting concentration of 20 ppm, along with conventional or high-frequency ventilation (HFV), if dual pulse oximetry or echocardiography demonstrates right-to-left shunt and the oxygen index is >25.[63][64] Echocardiographic evaluation is recommended before initiating iNO therapy to rule out cardiac disease, to assess pulmonary artery pressure and ventricular function. About 60% of infants with PPHN respond to iNO therapy.[63] iNO therapy in term and near-term infants with respiratory failure showed reduction in use of ECMO, improved oxygenation by 50%, and decreased oxygenation index by 15.1 within 30 to 60 minutes.[64]
In patients with severe respiratory distress at presentation, treatment consists of HFV plus iNO.
If there is no response to iNO therapy along with HFV, infants should be placed on ECMO. Indications include an alveolar arterial oxygen gradient >610 mmHg and an oxygen index ≥40.[65][66]
Arterial blood pressure must be maintained in suprasystemic pressure to overcome a right-to-left shunt at the ductal level secondary to PPHN. Though well researched, dopamine’s non-selective systemic and pulmonary vasoconstriction can often lead to increased pulmonary vascular resistance and studies now suggest the risk-benefit of alternate drugs may be superior in the treatment of PPHN.[61][62] Inotropes such as dobutamine and adrenaline (which is also a vasopressor) can be used in infants with MAS and PPHN to maintain higher systemic pressure and avoid hypotension from simultaneously giving sedatives.
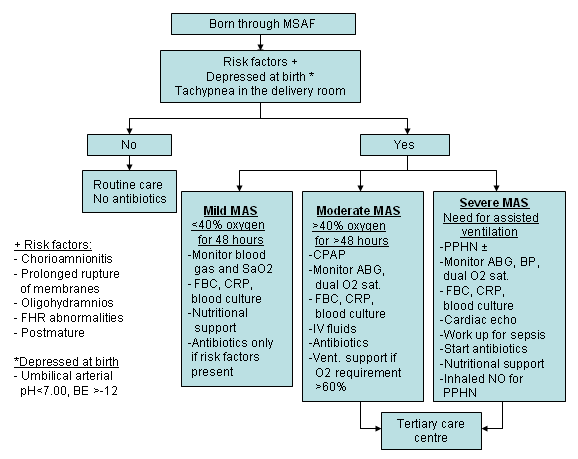
Treatment of acidosis with systemic alkalinisation using sodium bicarbonate may be considered, although this treatment is not commonly used due to the lack of studies establishing its benefit.[67] When sodium bicarbonate is used, physicians should be aware of complications of sodium bicarbonate in the absence of adequate ventilation and potential electrolyte imbalance (hypernatraemia).[Figure caption and citation for the preceding image starts]: Possible clinical presentations and management of infants born in meconium-stained amniotic fluid (MSAF). BE: base excess; CPAP: continuous positive airway pressure; FHR: fetal heart rate; IV: intravenous; NO: nitric oxide; PPHN: persistent pulmonary hypertension of the newbornCreated by Dr Vidyasagar and Dr Bhat [Citation ends].
Use of this content is subject to our disclaimer