History and exam
Key diagnostic factors
common
pallor
Nonspecific sign of anemia.
jaundice
Due to increased bilirubin from red blood cell destruction.
Other diagnostic factors
common
fatigue
Nonspecific sign of anemia.
shortness of breath
Nonspecific sign of anemia.
dizziness
Nonspecific sign of anemia.
splenomegaly
Nonspecific sign. May be present in hereditary spherocytosis, non-Hodgkin lymphoma, or portal hypertension due to cirrhosis.[38]
uncommon
active infections
Direct parasitic destruction of the red blood cell (RBC) caused by agents such as malaria, Babesia, or Bartonella.[32][33]
Infection-related toxins can produce direct lysis of RBC, as in clostridial sepsis.
Also associations with pneumococci, Escherichia coli, and staphylococci.
May be present in mild or localized infection as well as in systemic, severe infection.
episodic dark urine (hemoglobinuria)
Suggestive of paroxysmal nocturnal hemoglobinuria.
triggered by exposure to cold
Donath-Landsteiner antibody (cold agglutinins) may be present.
Risk factors
strong
autoimmune disorders
Systemic lupus erythematosus (SLE), rheumatoid arthritis, and scleroderma are associated with an increased incidence of warm autoimmune hemolytic anemia due to autoantibody production.[10][11] The incidence of clinically significant hemolytic anemia has been described in up to 10% of patients with SLE.[13] On a lesser scale, other entities such as rheumatoid arthritis, scleroderma, dermatomyositis, and inflammatory bowel disease have an increased incidence of autoantibody formation.[14]
lymphoproliferative disorders
Oncology patients, specifically patients with chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), and plasma cell disorders, often develop warm autoimmune hemolytic anemia (AIHA).[15][16][17][18][19]
Estimates of the incidence of AIHA are 7% to 10% in patients with CLL and 3% in patients with NHL.[15][16]
prosthetic heart valve
family origin in Mediterranean, Middle East, Africa, or Southeast Asia
Hemoglobinopathies (thalassemia, sickle cell anemia) are more common in groups originating in these areas of the world.[23][24]
Africa is the World Health Organization region with the highest incidence of glucose-6-phosphate dehydrogenase deficiency; it is very common in the sub-Saharan region.[3][4][Figure caption and citation for the preceding image starts]: Digitally-colorized scanning electron micrograph showing normal red blood cells (RBCs) and a sickle cell RBC (left) in a blood specimen of patient with sickle cell anemiaCDC/ Sickle Cell Foundation of Georgia: Jackie George, Beverly Sinclair [Citation ends].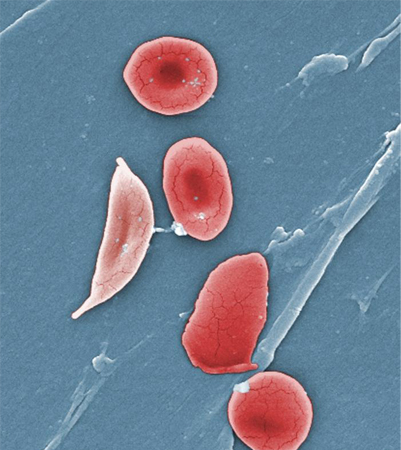
family history of hemoglobinopathy or red blood cell membrane defects
Suggests heritable causes of hemolysis, such as glucose-6-phosphate dehydrogenase deficiency, thalassemia, or sickle cell anemia.[1]
paroxysmal nocturnal hemoglobinuria
A rare disorder resulting in an acquired red blood cell membrane defect and subsequent hemolysis. Characterized by hemolysis, increased risk of thrombosis, and bone marrow failure.[25]
recent exposure to cephalosporins, penicillins, quinine derivatives, or nonsteroidal anti-inflammatory drugs
Many drugs have been implicated in immune responses resulting in hemolysis.[12][26] Cephalosporins in particular have been associated with significant hemolysis, including fatal cases.[27] If hemolytic anemia is diagnosed and drugs are suspected as the inciting event, a comprehensive search of the patient's medications should be conducted.
recent exposure to naphthalene or fava beans
May trigger hemolysis in glucose-6-phosphate dehydrogenase deficiency (G6PD).[28] Naphthalene is found in moth balls.
G6PD deficiency is the most common deficiency of erythrocyte enzymes that results in a shortening of the erythrocyte lifespan.
thermal injury
Can cause hemolysis by disruption of red cells by microangiopathic processes.
weak
exceptional exertion
Circulatory trauma may lead to footstrike (march) hemolysis.[29]
recent exposure to nitrites, dapsone, ribavirin, or phenazopyridine
Medications can induce nonimmune destruction of red blood cells, usually by oxidative stress.[30]
recent paraquat ingestion
Herbicides can induce nonimmune destruction of red blood cells, usually by oxidative stress.[31]
malaria
Direct interaction between the infective organism and the red blood cell is responsible for hemolysis.[32][Figure caption and citation for the preceding image starts]: A photomicrograph of a blood smear showing erythrocytes containing developing Plasmodium vivax parasitesCDC/ Dr Mae Melvin [Citation ends].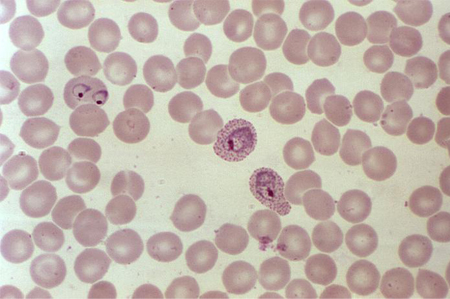
babesiosis
Direct interaction between the infective organism and the red blood cell is responsible for hemolysis.[33][Figure caption and citation for the preceding image starts]: Photomicrograph revealing the presence of what were determined to be numbers of intraerythrocytic Babesia sp. ring-form parasitesCDC/ Dr Mae Melvin [Citation ends].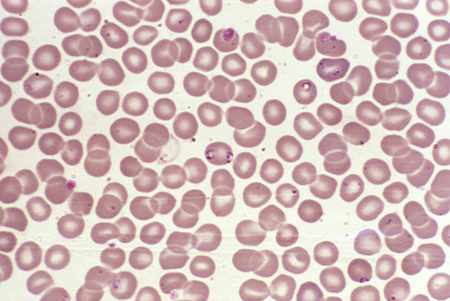
bartonellosis
Direct interaction between the infective organism and the red blood cell is responsible for hemolysis.
leishmaniasis
Direct interaction between the infective organism and the red blood cell is responsible for hemolysis.
Clostridium perfringens infection
Haemophilus influenzae type B infection
Capsular polysaccharide binds with the red blood cell membrane, and infected patients form antibodies to this complex.[36]
liver disease
May be associated with splenomegaly and hemolysis. Alcoholic cirrhosis in particular results in structural changes to the red blood cell membrane and subsequent hemolysis.
Use of this content is subject to our disclaimer