Etiology
Estrogen stimulates breast duct development in both men and women in the presence of permissive levels of growth hormone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) from the pituitary. Progesterone stimulates development of breast alveoli. Androgens oppose estrogen action. Prolactin is not a growth factor for the breast in men, but high prolactin levels suppress the hypothalamic generator of gonadotropin-releasing hormone (GnRH) pulses that stimulate production of LH and FSH. Gynecomastia results from a relative excess of estrogen or estrogen action or a relative deficiency of androgen or androgen action. [Figure caption and citation for the preceding image starts]: Hormones involved in male breast developmentFrom the collection of Catherine B. Niewoehner, MD [Citation ends].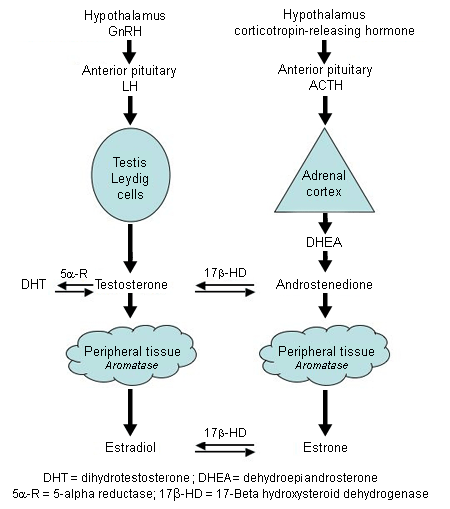
In men, 95% of testosterone is produced in the Leydig cells of the testes in response to LH. The remaining 5% of testosterone and the weak androgen androstenedione are produced by the adrenal glands. Testosterone can be converted to the more potent androgen dihydrotestosterone by the enzyme 5-alpha reductase. Testosterone is converted to estrogen by the enzyme aromatase, which also converts androstenedione to the weak estrogen estrone. Aromatase is found predominantly in adipose tissue. In the blood, 50% to 60% of testosterone is tightly bound to sex hormone binding globulin.[17] Most of the rest is weakly bound to albumin and is considered bioavailable. Only the small free testosterone fraction (0.5% to 3%) is active. Free testosterone declines slowly with age.[18][19] Estrogen is less strongly bound to sex hormone binding globulin than testosterone. Defects in any of these pathways can cause gynecomastia.[20][21]
Approximately 10% to 20% of gynecomastia is believed to be attributable to prescribed drugs; many medications have been implicated, but the quality of the evidence is often very poor.[22][23][24][25]
Causes of elevated estrogen levels or activity:
Excess aromatase activity: obesity, congenital activating mutation of aromatase, tumors (Sertoli cell, Leydig cell, germ cell)[26]
Excess androgen converted to estrogen: testicular feminization, anabolic steroid use, excessive androgen replacement therapy, cirrhosis
Differences in sex development: coexisting functional ovarian and testicular tissue (along with estrogen production from ovarian tissue)
Chronic illness or starvation and refeeding: more profound suppression of testosterone than estrogen with fasting, and earlier increase in estrogen than testosterone with refeeding
Estrogen-producing tumors: Sertoli cell, Leydig cell, germ cell, human chorionic gonadotropin (hCG)-producing tumors (stimulate Leydig cell estrogen production), feminizing adrenal adenoma and carcinoma[27]
Exogenous estrogen exposure
Drugs that stimulate estrogen receptors: diethylstilbestrol, digitalis, phenytoin
Hyperthyroidism.
Causes of testosterone deficiency:
Hypothalamic: hypogonadotropic hypogonadism (Kallmann syndrome, the failure of GnRH-producing neurons)
Hyperprolactinemic: pituitary tumor, hypothyroidism, renal disease (GnRH pulse generator suppression)
Normoprolactinemic pituitary disease or tumor (decrease in gonadotropin production)
Differences in sex development: coexisting functional ovarian and testicular tissue (along with estrogen production from ovarian tissue), Klinefelter syndrome (47XXY)
Testicular: castration, infection (e.g., orchitis), infiltration (e.g., hemochromatosis), chemotherapy- or radiation-related damage to Leydig cells, neurologic diseases (spinal cord injury, myotonic dystrophy)
Medications: gonadotropin-releasing hormone (GnRH) agonists, cancer chemotherapeutic agents, ketoconazole, metronidazole, spironolactone, some antipsychotic agents.
Causes of impaired testosterone action:
Aging
Elevated estrogen levels
Genetic factors
Medications: anticonvulsants, androgen receptor blockers (bicalutamide, flutamide), spironolactone, 5-alpha reductase inhibitors, H2 antagonists (e.g., cimetidine), proton-pump inhibitors (impair testosterone action less than H2 antagonists).[28][29][30][31]
Pathophysiology
Estrogen excess or increased estrogen sensitivity stimulates proliferation of breast ducts and ductal epithelium. If androgen deficiency or androgen inhibition is present, the effect of estrogen is more pronounced, even if the estrogen level is normal. At puberty, marked increases in growth hormone, insulin-like growth factor 1, follicle-stimulating hormone, and luteinizing hormone drive both estrogen and testosterone production, but the estrogen peak precedes the peak in testosterone production. There are many contributors to increased estrogen versus androgen effect in adults. Regardless of cause, the initial phase with proliferating ducts, ductal epithelium, stroma, and fibroblasts, accompanied by inflammation and edema, often gives way to a more quiescent stage characterized by dilated ducts, stroma, and fibrosis with little inflammation. The fibrotic stage is far less likely to regress either spontaneously or with treatment. Progesterone levels in men are low, so there is little alveolar tissue in the male breast even when gynecomastia is present. Hence, galactorrhea is rare.[Figure caption and citation for the preceding image starts]: Histology: normal male breast; rare, isolated ducts; no lobules; 10X magnificationFrom Minneapolis Veterans Affairs Medical Center pathology collection [Citation ends].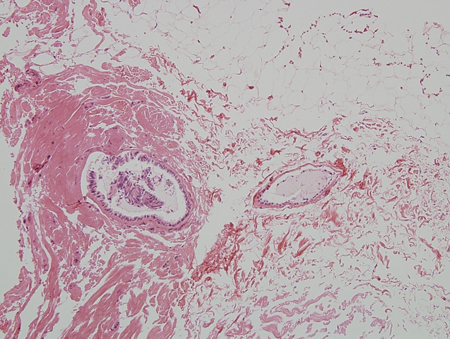 [Figure caption and citation for the preceding image starts]: Histology: gynecomastia; clusters of ducts, halos of edema, fibrous background; 5X magnificationFrom the collection of Catherine B. Niewoehner, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: Histology: gynecomastia; clusters of ducts, halos of edema, fibrous background; 5X magnificationFrom the collection of Catherine B. Niewoehner, MD [Citation ends].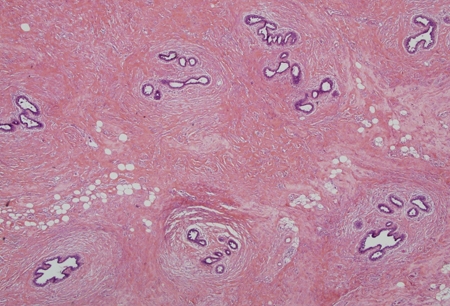 [Figure caption and citation for the preceding image starts]: Histology: normal female breast; many ducts; prominent lobules; 10X magnificationFrom Minneapolis Veterans Affairs Medical Center pathology collection [Citation ends].
[Figure caption and citation for the preceding image starts]: Histology: normal female breast; many ducts; prominent lobules; 10X magnificationFrom Minneapolis Veterans Affairs Medical Center pathology collection [Citation ends].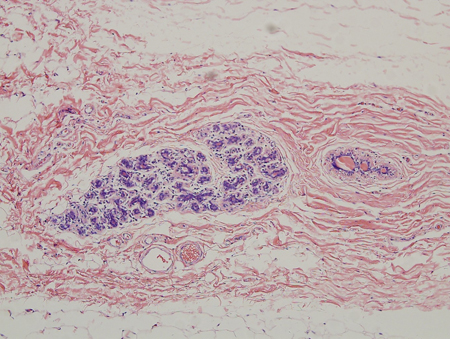
Classification
Clinical categories[1]
There is no formal classification scheme. However, there are clinically accepted categories.
Newborn gynecomastia:
A physiologic response to high levels of maternal and placental estrogen transferred in utero. Neonatal milk secretion can often occur.
Pubertal gynecomastia:
A physiologic response to the 30-fold increase in testosterone fueled by marked increases in growth hormone, insulin-like growth factor-1, follicle-stimulating hormone, and luteinizing hormone at puberty
Estrogen increases 3-fold, but peaks earlier than testosterone.
Adult gynecomastia (physiologic):
A physiologic response to the decrease in free testosterone and the increase in adipose tissue that often accompanies aging
Testosterone is converted to estrogen in adipose tissue.
Adult gynecomastia (nonphysiologic):
A response to increased estrogen effect relative to androgen effect from a cause other than aging.
Pseudogynecomastia:
Male breast enlargement entirely due to adipose tissue.
Simon classification[2]
Based on the amount of tissue to be removed in cases of surgery:
Class I: minor breast enlargement with no skin redundancy
Class IIa: moderate breast enlargement with no skin redundancy
Class IIb: moderate breast enlargement with minor skin redundancy
Class III: marked breast enlargement with major skin redundancy (resembles the female breast).
Use of this content is subject to our disclaimer