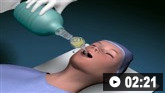
Initial management options include a combination of oxygen, diuretics, vasodilators, inotropes, and vasopressors.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Other possible therapies include extracorporeal ultrafiltration; ventilation (noninvasive and endotracheal intubation); and mechanical circulatory support (e.g., intra-aortic balloon pump, ventricular assist devices).[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
Evidence supporting the use of intravenous morphine to treat dyspnea is lacking and data suggest there might be adverse effects.[64]Lin Y, Chen Y, Yuan J, et al. Intravenous morphine use in acute heart failure increases adverse outcomes: a meta-analysis. Rev Cardiovasc Med. 2021 Sep 24;22(3):865-72.
https://www.imrpress.com/journal/RCM/22/3/10.31083/j.rcm2203092/htm
http://www.ncbi.nlm.nih.gov/pubmed/34565084?tool=bestpractice.com
[65]Pratama NR, Anastasia ES, Wardhani NP, et al. Clinical outcomes of opioid administration in acute and chronic heart failure: a meta-analysis. Diabetes Metab Syndr. 2022 Oct;16(10):102636.
https://www.sciencedirect.com/science/article/pii/S1871402122002508?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/36240686?tool=bestpractice.com
Hence the current recommendation is that morphine should not be used routinely in patients with acute heart failure.[36]Ezekowitz JA, O'Meara E, McDonald MA, et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017 Nov;33(11):1342-433.
https://www.onlinecjc.ca/article/S0828-282X(17)30973-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29111106?tool=bestpractice.com
Morphine can be used for palliative care, and is helpful in individual patients because of its venodilator properties and because it decreases sympathetic drive; however, it should be used cautiously as it can cause respiratory depression, potentially increasing the chance of mechanical ventilation.
All patients should be admitted to the hospital. If the patient responds adequately to initial treatment and meets strict selection criteria, telemetry monitoring may be acceptable (e.g., hospital-at-home program).[66]Writing Committee; Hollenberg SM, Stevenson LW, Ahmad T, et al. 2024 ACC expert consensus decision pathway on clinical assessment, management, and trajectory of patients hospitalized with heart failure focused update: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2024 Sep 24;84(13):1241-67.
https://www.sciencedirect.com/science/article/pii/S0735109724074497
http://www.ncbi.nlm.nih.gov/pubmed/39127954?tool=bestpractice.com
Those who are hypotensive or fail to respond to initial therapy require admission to the intensive care unit and may need invasive monitoring if tissue perfusion is compromised.[67]McKelvie RS, Benedict CR, Yusuf S. Evidence based cardiology: prevention of congestive heart failure and management of asymptomatic left ventricular dysfunction. BMJ. 1999 May 22;318(7195):1400-2.
http://www.ncbi.nlm.nih.gov/pubmed/10334754?tool=bestpractice.com
If cardiogenic shock is present, invasive evaluation is required.
Patients with acute heart failure should undergo evaluation for potential precipitating factors, including myocardial ischemia, arrhythmias (commonly atrial fibrillation), underlying valvular disease, exacerbation of hypertension, anemia, thyroid disorders, and drug interactions. Other concomitant conditions, such as pneumonia and pulmonary embolism, may also be contributing factors.
Venous thromboembolism prophylaxis is recommended in all patients. See Venous thromboembolism (VTE) prophylaxis.
Patients with acute heart failure with reduced ejection fraction who are iron deficient should receive intravenous iron supplementation to reduce the risk of future heart failure hospitalizations.[68]McDonagh TA, Metra M, Adamo M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023 Oct 1;44(37):3627-39.
https://academic.oup.com/eurheartj/article/44/37/3627/7246292
http://www.ncbi.nlm.nih.gov/pubmed/37622666?tool=bestpractice.com
[69]Hamza M, Sattar Y, Manasrah N, et al. Meta-analysis of efficacy and safety of intravenous iron in patients with iron deficiency and heart failure with reduced ejection fraction. Am J Cardiol. 2023 Sep 1;202:119-30.
http://www.ncbi.nlm.nih.gov/pubmed/37429060?tool=bestpractice.com
Maintenance of oxygen saturation
High-flow oxygen is recommended in patients with a capillary oxygen saturation <90% or PaO₂ <60 mmHg (8.0 kPa) to correct hypoxemia.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
Ventilation with noninvasive positive pressure ventilation or continuous positive airway pressure may be required if oxygen saturation cannot be maintained by oxygenation alone, and is associated with a decreased requirement for intubation and mechanical ventilation.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
Mechanical ventilation is only used when other treatments, including noninvasive ventilation methods, fail.
Hemodynamically stable
Diuretics and vasodilators
Loop diuretics are the mainstay of treatment for patients who are hemodynamically stable and are effective in relieving symptoms.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[73]Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011 Mar 3;364(9):797-805.
http://www.ncbi.nlm.nih.gov/pubmed/21366472?tool=bestpractice.com
Loop diuretics used for the treatment of acute heart failure and congestion include furosemide, bumetanide, and torsemide. The most commonly used agent appears to be furosemide, but some patients may respond more favorably to another loop diuretic.
Intravenous diuretics (bolus or continuous infusion) are indicated on initial hospitalization in patients with pulmonary congestion and volume overload.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
All patients with symptoms and signs of congestion should receive diuretics, irrespective of the left ventricular ejection fraction. Patients already taking oral loop diuretics should be started on a higher dose intravenously (may require double their usual dose), with further titration as needed.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[66]Writing Committee; Hollenberg SM, Stevenson LW, Ahmad T, et al. 2024 ACC expert consensus decision pathway on clinical assessment, management, and trajectory of patients hospitalized with heart failure focused update: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2024 Sep 24;84(13):1241-67.
https://www.sciencedirect.com/science/article/pii/S0735109724074497
http://www.ncbi.nlm.nih.gov/pubmed/39127954?tool=bestpractice.com
Diuretic response should be evaluated shortly after start of diuretic therapy, initially with hourly urine output measurement; ongoing monitoring should include careful measurement of 24-hour fluid intake and output, vital signs, and standing body weight measured at the same time each day.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Nonloop diuretics may be added if there is an inadequate response to loop diuretics alone.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Evidence from randomized controlled trials supports the use of acetazolamide and hydrochlorothiazide as add-on diuretic therapy; other options include metolazone, or acute use of aldosterone antagonists such as spironolactone and eplerenone.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[74]Trullàs JC, Morales-Rull JL, Casado J, et al. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J. 2023 Feb 1;44(5):411-21.
http://www.ncbi.nlm.nih.gov/pubmed/36423214?tool=bestpractice.com
[75]Mullens W, Dauw J, Martens P, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022 Sep 29;387(13):1185-95.
https://www.nejm.org/doi/10.1056/NEJMoa2203094
http://www.ncbi.nlm.nih.gov/pubmed/36027559?tool=bestpractice.com
Acetazolamide added to loop diuretic therapy in patients with acute decompensated heart failure results in a greater incidence of successful decongestion.[75]Mullens W, Dauw J, Martens P, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022 Sep 29;387(13):1185-95.
https://www.nejm.org/doi/10.1056/NEJMoa2203094
http://www.ncbi.nlm.nih.gov/pubmed/36027559?tool=bestpractice.com
Hydrochlorothiazide with intravenous furosemide results in greater diuresis and weight loss compared to furosemide alone, but with worsening renal function.[74]Trullàs JC, Morales-Rull JL, Casado J, et al. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J. 2023 Feb 1;44(5):411-21.
http://www.ncbi.nlm.nih.gov/pubmed/36423214?tool=bestpractice.com
Careful monitoring of renal function and electrolytes is essential when loop and nonloop diuretics are used in combination.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
The minimum dose of diuretics should be used to relieve congestion, keep the patient asymptomatic, and maintain a dry weight (defined as when the patient is euvolemic).
In patients with reduced left ventricular ejection fraction, diuretics should be used only in combination with other medical therapies, such as an ACE inhibitor (or an angiotensin-II receptor antagonist or an angiotensin-II receptor antagonist/neprilysin inhibitor), a beta-blocker, and an aldosterone antagonist.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
In both acute heart failure and stable congestive heart failure, loop diuretics are the preferred agent for most patients. However, a thiazide diuretic may be considered for patients with hypertension and only mild fluid retention.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Vasodilators (nitroglycerin, nitroprusside) are often used in acute heart failure to relieve symptoms of pulmonary congestion in patients without systemic hypotension; however, they do not improve long-term outcomes (i.e., reduction of mortality or rehospitalization).[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Nitroglycerin is preferred in emergency settings.[34]American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes. Jun 2022 [internet publication].
https://www.acep.org/globalassets/new-pdfs/clinical-policies/acute-heart-failure-syndrome-clinical-policy.pdf
Nitroprusside is usually given in the intensive care setting where enhanced monitoring is available (e.g., invasive hemodynamic blood pressure monitoring), due to its potential for marked hypotension and risk of cyanide toxicity.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
In patients who do not respond to initial therapy, extracorporeal ultrafiltration is used to reduce volume overload.[76]Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007 Feb 13;49(6):675-83.
http://www.onlinejacc.org/content/49/6/675
http://www.ncbi.nlm.nih.gov/pubmed/17291932?tool=bestpractice.com
[77]Srivastava M, Harrison N, Caetano AFS, et al. Ultrafiltration for acute heart failure. Cochrane Database Syst Rev. 2022 Jan 21;1(1):CD013593.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013593.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/35061249?tool=bestpractice.com
Hemodynamically unstable
Patients with hypotension (i.e., systolic blood pressure [BP] <90 mmHg) or shock should receive oxygen therapy if capillary oxygen saturation <90% or PaO₂ <60 mmHg (8.0 kPa), vasoactive drugs, and ventilation and mechanical circulatory support if needed.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
Cardiogenic shock is characterized by critical reduction in cardiac output and end-organ hypoperfusion in a patient with a systolic BP <90 mmHg.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Short-term intravenous infusion of a vasoactive agent (vasopressor and/or inotrope) should be considered in patients with hypotension (systolic BP <90 mmHg) and/or signs or symptoms of hypoperfusion, despite adequate filling status.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Vasoactive agents may cause tachycardia, and induce arrhythmias and myocardial ischemia.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Vasopressor therapy aims to reverse the mismatch between vessel tone and intravascular volume by inducing vasoconstriction. The preferred vasopressor is norepinephrine (noradrenaline).[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Inotropes (e.g., dobutamine, milrinone) can increase cardiac output and improve hemodynamics in patients with cardiogenic shock.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Inotropes should be used with caution because there is evidence that they result in increased mortality.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[13]Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005 Jul 5;46(1):57-64.
http://www.ncbi.nlm.nih.gov/pubmed/15992636?tool=bestpractice.com
Inotropes should be discontinued if there are sustained arrhythmias or symptomatic coronary ischemia. Continuous monitoring of cardiac rhythm is recommended during infusion of inotropes. There is a lack of robust evidence to suggest a clear benefit of one inotropic agent over another in cardiogenic shock.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[78]Kivikko M, Pollesello P, Tarvasmäki T, et al. Effect of baseline characteristics on mortality in the SURVIVE trial on the effect of levosimendan vs dobutamine in acute heart failure: Sub-analysis of the Finnish patients. Int J Cardiol. 2016 Jul 15;215:26-31.
https://www.doi.org/10.1016/j.ijcard.2016.04.064
http://www.ncbi.nlm.nih.gov/pubmed/27107540?tool=bestpractice.com
Selection of appropriate vasoactive agents may vary according to clinician preference and local practice guidelines. Consult a specialist for guidance on suitable regimens.
Temporary mechanical circulatory support (MCS) devices (e.g., extracorporeal membrane oxygenation or intra-aortic balloon pump) should be considered in patients with persistent cardiogenic shock despite inotropic therapy.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[79]Geller BJ, Sinha SS, Kapur NK, et al. Escalating and de-escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2022 Aug 9;146(6):e50-68.
https://www.doi.org/10.1161/CIR.0000000000001076
http://www.ncbi.nlm.nih.gov/pubmed/35862152?tool=bestpractice.com
See Shock.
Specific treatment of underlying cause
Coronary artery disease
Intravenous nitroglycerin is first-line treatment.
The common adverse effect of nitroglycerin is headache and hypotension. The dose of nitrates should be reduced if systolic BP decreases below 90 to 100 mmHg, and discontinued permanently if BP drops further.
In cases of significant coronary artery disease causing acute heart failure, percutaneous revascularization or coronary artery bypass should be carried out. Aspirin, in combination with a P2Y12 inhibitor (e.g., clopidogrel, prasugrel, ticagrelor), is given to all patients with coronary ischemia and those undergoing revascularization.[80]Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014 Dec 23;130(25):e344-426.
https://www.doi.org/10.1161/CIR.0000000000000134
http://www.ncbi.nlm.nih.gov/pubmed/25249585?tool=bestpractice.com
In the case of cardiogenic shock with acute myocardial infarction, revascularization is recommended. Thrombolysis in this setting is not effective.[81]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jul 1;82(1):E1-27.
http://circ.ahajournals.org/content/127/4/e362.long
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
See Overview of acute coronary syndrome.
Hypertensive emergency
Use of intravenous nitroglycerin is recommended.
If additional medications are needed, nitroprusside is recommended in addition to other choices. Nitroprusside should be given in a setting where enhanced monitoring is available (e.g., invasive hemodynamic blood pressure monitoring).
See Hypertensive emergencies.
Cardiac arrhythmias
Either rapid arrhythmias or severe bradycardia/conduction disturbance can precipitate acute heart failure.
Patients presenting acutely with atrial fibrillation or atrial flutter require anticoagulation, rate control, and rhythm control when indicated (e.g., urgent DC cardioversion for patients with hemodynamic instability).[82]Reddy YNV, Borlaug BA, Gersh BJ. Management of atrial fibrillation across the spectrum of heart failure with preserved and reduced ejection fraction. Circulation. 2022 Jul 26;146(4):339-57.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.122.057444
http://www.ncbi.nlm.nih.gov/pubmed/35877831?tool=bestpractice.com
Severe bradycardia may require temporary pacing or drug interventions; patients with non-reversible causes may require an implantable pacemaker with or without a defibrillator.[83]Chung MK, Patton KK, Lau CP, et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm. 2023 Sep;20(9):e17-91.
https://www.heartrhythmjournal.com/article/S1547-5271(23)02026-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37283271?tool=bestpractice.com
See Evaluation of tachycardia and Bradycardia.
Valvular heart disease
In cases of severe aortic stenosis with heart failure, nitroprusside can be used with careful monitoring, provided the patient is not hypotensive.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[84]Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med. 2003 May 1;348(18):1756-63.
http://www.nejm.org/doi/full/10.1056/NEJMoa022021#t=article
http://www.ncbi.nlm.nih.gov/pubmed/12724481?tool=bestpractice.com
The definitive treatment for aortic stenosis or mitral stenosis is valve replacement, but in resistant heart failure a percutaneous valvotomy may be used as temporary measure until definitive valve replacement is carried out. In mitral stenosis, percutaneous valvuloplasty may be done if no thrombus is present on transesophageal echocardiogram.
Similarly in heart failure associated with mitral regurgitation or aortic regurgitation, a vasodilating drug such as nitroprusside may be used.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
A decrease in the peripheral arterial resistance results in an increase in the cardiac output and a decrease in regurgitant volume, which, in turn, is associated with a reduction in left ventricular end-diastolic volume and an augmentation of the ejection fraction.
See Aortic stenosis, Aortic regurgitation, Mitral stenosis, and Mitral regurgitation.
Acute right heart failure
Therapy is centered around treatment of the underlying pathology; e.g., pulmonary embolism (anticoagulation, thrombolytics, catheterization, or surgically directed thrombectomy), right ventricular infarction (percutaneous coronary intervention or thrombolytics), and chronic thromboembolic pulmonary hypertension (thromboendarterectomy).[85]Lahm T, McCaslin CA, Wozniak TC, et al. Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol. 2010 Oct 26;56(18):1435-46.
http://www.ncbi.nlm.nih.gov/pubmed/20951319?tool=bestpractice.com
See Pulmonary embolism.
Acute myocarditis
Myocarditis caused by autoimmune disease (clinical or endomyocardial biopsy evidence of autoimmune disease) including giant cell myocarditis is treated with single or combination immunosuppressant therapy which may include corticosteroids, azathioprine, and cyclosporine.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[86]Cooper LT Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis - natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med. 1997 Jun 26;336(26):1860-6.
http://www.nejm.org/doi/full/10.1056/NEJM199706263362603#t=article
http://www.ncbi.nlm.nih.gov/pubmed/9197214?tool=bestpractice.com
Treatment of other forms of myocarditis is limited to supportive care, alongside standard heart failure therapy for at least 6 months.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
See Myocarditis.
Resistance to maximal medical therapy
In cases of advanced heart failure resistant and refractory to maximal medical therapy, a durable mechanical circulatory support (MCS) device (e.g., a left ventricular assist device [LVAD]) is recommended for select patients (e.g., patients dependent on continuous intravenous inotropes).[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[87]Peura JL, Colvin-Adams M, Francis GS, et al; American Heart Association. Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012 Nov 27;126(22):2648-67.
http://circ.ahajournals.org/content/126/22/2648.long
http://www.ncbi.nlm.nih.gov/pubmed/23109468?tool=bestpractice.com
There are several accepted indications for implantation of a durable LVAD, including bridge to transplantation and destination therapy (permanent pump implantation in patients not eligible for cardiac transplantation).[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[88]Gopinathannair R, Cornwell WK, Dukes JW, et al. Device therapy and arrhythmia management in left ventricular assist device recipients: a scientific statement from the American Heart Association. Circulation. 2019 May 14;139(20):e967-89.
https://www.doi.org/10.1161/CIR.0000000000000673
http://www.ncbi.nlm.nih.gov/pubmed/30943783?tool=bestpractice.com
Some of the absolute contraindications for providing durable mechanical support include irreversible hepatic, renal, and neurologic disease; medical nonadherence; and severe psychosocial limitations.[89]Cook JL, Colvin M, Francis GS, et al. Recommendations for the use of mechanical circulatory support: ambulatory and community patient care: a scientific statement from the American Heart Association. Circulation. 2017 Jun 20;135(25):e1145-58.
https://www.doi.org/10.1161/CIR.0000000000000507
http://www.ncbi.nlm.nih.gov/pubmed/28559233?tool=bestpractice.com
In some cases of nonischemic cardiomyopathy, sustained reversal of severe heart failure is seen with implantation of an LVAD.[90]Birks EJ, Tansley PD, Hardy J, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006 Nov 2;355(18):1873-84.
http://www.nejm.org/doi/full/10.1056/NEJMoa053063#t=article
http://www.ncbi.nlm.nih.gov/pubmed/17079761?tool=bestpractice.com
The use of LVADs has evolved significantly over the past 25 years and various types of LVAD now exist.
Use of temporary devices can help stabilize patients and allow time for decisions about the appropriateness of transitions to definitive management (bridge to decision), such as durable MCS or cardiac transplantation.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Extracorporeal devices, the most common of which are the extracorporeal membrane oxygenators, require full heparinization and are typically used for days or weeks as a bridge for patients who are expected to recover within days. Percutaneous short-term devices (e.g., Tandem Heart) are inserted through the femoral artery and advanced into the left ventricle. Longer-term assist devices are divided into first-generation (e.g., Heart Mate I), second-generation (e.g., Heart Mate II), and third-generation (e.g., HVAD and Dura Heart) devices.
Ongoing therapy
Once the patient is stabilized, definitive medical therapy for heart failure should be commenced.
Recommended therapies include:[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Renin-angiotensin system inhibitors (i.e., angiotensin-II receptor antagonist/neprilysin inhibitor, ACE inhibitor, or an angiotensin-II receptor antagonist)
Beta-blockers
Aldosterone antagonists
Sodium-glucose cotransporter 2 (SGLT2) inhibitors.
For patients with heart failure with reduced ejection fraction (HFrEF), a combination of drugs from all four of these medication classes should be commenced, increased rapidly to maximum recommended and tolerated doses, and continued long term.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[68]McDonagh TA, Metra M, Adamo M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023 Oct 1;44(37):3627-39.
https://academic.oup.com/eurheartj/article/44/37/3627/7246292
http://www.ncbi.nlm.nih.gov/pubmed/37622666?tool=bestpractice.com
[91]Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022 Dec 3;400(10367):1938-52.
http://www.ncbi.nlm.nih.gov/pubmed/36356631?tool=bestpractice.com
Patients who have persistent signs of fluid overload will need ongoing diuretics.
Usually an angiotensin-II receptor antagonist/neprilysin inhibitor (e.g., sacubitril/valsartan) or an ACE inhibitor (or an angiotensin-II receptor antagonist if ACE inhibitors are not tolerated and use of an angiotensin-II receptor antagonist/neprilysin inhibitor is not feasible) is started first, followed by the addition of beta-blockers.
[  ]
How do angiotensin receptor blockers (ARBs) affect outcomes in people with heart failure?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.298/fullShow me the answer An angiotensin-II receptor antagonist/neprilysin inhibitor is recommended both as a first-line treatment for patients newly diagnosed with acute heart failure, and to replace ACE inhibitor (or angiotensin-II receptor antagonist) therapy in patients with chronic heart failure with reduced ejection fraction that remains symptomatic despite existing therapy.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
The dose of these agents should be increased to the maximum tolerated dose depending upon BP and heart rate. Typically, beta-blockers are started only after patients have stabilized, but should continue long term to reduce the risk of major cardiovascular events even if symptoms do not improve.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
]
How do angiotensin receptor blockers (ARBs) affect outcomes in people with heart failure?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.298/fullShow me the answer An angiotensin-II receptor antagonist/neprilysin inhibitor is recommended both as a first-line treatment for patients newly diagnosed with acute heart failure, and to replace ACE inhibitor (or angiotensin-II receptor antagonist) therapy in patients with chronic heart failure with reduced ejection fraction that remains symptomatic despite existing therapy.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
The dose of these agents should be increased to the maximum tolerated dose depending upon BP and heart rate. Typically, beta-blockers are started only after patients have stabilized, but should continue long term to reduce the risk of major cardiovascular events even if symptoms do not improve.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Patients with ongoing symptoms despite this therapy should be treated as having chronic heart failure. In patients with reduced left ventricular ejection fraction (LVEF), an aldosterone antagonist (e.g., spironolactone, eplerenone) should be prescribed.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Aldosterone antagonists require careful monitoring of potassium, renal function, and diuretic dosing to minimize risk of hyperkalemia and renal insufficiency.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
There is also evidence to support the long-term use of a SGLT2 inhibitor (e.g., dapagliflozin, empagliflozin, sotagliflozin) in patients with reduced LVEF regardless of whether they have type 2 diabetes mellitus.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[66]Writing Committee; Hollenberg SM, Stevenson LW, Ahmad T, et al. 2024 ACC expert consensus decision pathway on clinical assessment, management, and trajectory of patients hospitalized with heart failure focused update: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2024 Sep 24;84(13):1241-67.
https://www.sciencedirect.com/science/article/pii/S0735109724074497
http://www.ncbi.nlm.nih.gov/pubmed/39127954?tool=bestpractice.com
[92]Usman MS, Siddiqi TJ, Anker SD, et al. Effect of SGLT2 inhibitors on cardiovascular outcomes across various patient populations. J Am Coll Cardiol. 2023 Jun 27;81(25):2377-87.
http://www.ncbi.nlm.nih.gov/pubmed/37344038?tool=bestpractice.com
[93]Carvalho PEP, Veiga TMA, Simões E Silva AC, et al. Cardiovascular and renal effects of SGLT2 inhibitor initiation in acute heart failure: a meta-analysis of randomized controlled trials. Clin Res Cardiol. 2023 Aug;112(8):1044-55.
https://link.springer.com/article/10.1007/s00392-022-02148-2
http://www.ncbi.nlm.nih.gov/pubmed/36592186?tool=bestpractice.com
Patients with diabetes taking SGLT2 inhibitors are at increased risk of developing diabetic ketoacidosis (including euglycemic ketoacidosis).[30]Musso G, Saba F, Cassader M, et al. Diabetic ketoacidosis with SGLT2 inhibitors. BMJ. 2020 Nov 12;371:m4147.
http://www.ncbi.nlm.nih.gov/pubmed/33184044?tool=bestpractice.com
For black patients with low LVEF, a combination of hydralazine and isosorbide dinitrate can be particularly beneficial, and can be considered for other patients unable to take first-line agents.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Patients with heart failure with mildly reduced ejection fraction (HFmrEF, LVEF 41% to 49%) should have repeat evaluation of LVEF to determine the trajectory of their disease process.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
For patients with HFmrEF and heart failure with preserved ejection fraction (HFpEF, LVEF ≥50%), good control of BP and other comorbidities (e.g., arrhythmias and underlying ischemia) is essential.[94]Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2023 May 9;81(18):1835-78.
https://www.sciencedirect.com/science/article/pii/S0735109723050982?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/37137593?tool=bestpractice.com
There is increasing evidence that therapies usually recommended for patients with reduced ejection fraction also benefit patients with HFmrF and HFpEF (LVEF >40%); in particular, SGLT2 inhibitors reduce future heart failure admissions regardless of ejection fraction and should be started in patients with heart failure before discharge when possible.[1]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
http://www.ncbi.nlm.nih.gov/pubmed/34447992?tool=bestpractice.com
[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[66]Writing Committee; Hollenberg SM, Stevenson LW, Ahmad T, et al. 2024 ACC expert consensus decision pathway on clinical assessment, management, and trajectory of patients hospitalized with heart failure focused update: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2024 Sep 24;84(13):1241-67.
https://www.sciencedirect.com/science/article/pii/S0735109724074497
http://www.ncbi.nlm.nih.gov/pubmed/39127954?tool=bestpractice.com
[68]McDonagh TA, Metra M, Adamo M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023 Oct 1;44(37):3627-39.
https://academic.oup.com/eurheartj/article/44/37/3627/7246292
http://www.ncbi.nlm.nih.gov/pubmed/37622666?tool=bestpractice.com
[92]Usman MS, Siddiqi TJ, Anker SD, et al. Effect of SGLT2 inhibitors on cardiovascular outcomes across various patient populations. J Am Coll Cardiol. 2023 Jun 27;81(25):2377-87.
http://www.ncbi.nlm.nih.gov/pubmed/37344038?tool=bestpractice.com
[94]Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2023 May 9;81(18):1835-78.
https://www.sciencedirect.com/science/article/pii/S0735109723050982?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/37137593?tool=bestpractice.com
[95]Packer M, Butler J, Zannad F, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-Preserved trial. Circulation. 2021 Oct 19;144(16):1284-94.
https://www.doi.org/10.1161/CIRCULATIONAHA.121.056824
http://www.ncbi.nlm.nih.gov/pubmed/34459213?tool=bestpractice.com
[96]Desai AS, Jhund PS, Claggett BL, et al. Effect of dapagliflozin on cause-specific mortality in patients with heart failure across the spectrum of ejection fraction: a participant-level pooled analysis of DAPA-HF and DELIVER. JAMA Cardiol. 2022 Dec 1;7(12):1227-34.
https://jamanetwork.com/journals/jamacardiology/fullarticle/2796866
http://www.ncbi.nlm.nih.gov/pubmed/36189985?tool=bestpractice.com
[97]Xiang B, Zhang R, Wu X, et al. Optimal pharmacologic treatment of heart failure with preserved and mildly reduced ejection fraction: a meta-analysis. JAMA Netw Open. 2022 Sep 1;5(9):e2231963.
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2796545
http://www.ncbi.nlm.nih.gov/pubmed/36125813?tool=bestpractice.com
If needed, diuretics may be prescribed to reduce congestion and improve symptoms.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
Treatment with ivabradine in stable patients with chronic heart failure (i.e., LVEF <35%) and a resting heart rate of >70 bpm - on a background of guideline-based heart failure therapy - is associated with reducing the risk of hospitalization for worsening heart failure.[2]Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-1032.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063
http://www.ncbi.nlm.nih.gov/pubmed/35363499?tool=bestpractice.com
[98]Borer JS, Böhm M, Ford I, et al; SHIFT Investigators. Effect of ivabradine on recurrent hospitalization for worsening heart failure in patients with chronic systolic heart failure: the SHIFT Study. Eur Heart J. 2012 Nov;33(22):2813-20.
http://eurheartj.oxfordjournals.org/content/33/22/2813.long
http://www.ncbi.nlm.nih.gov/pubmed/22927555?tool=bestpractice.com
In one randomized, double-blind, placebo-controlled trial, addition of ivabradine to standard background therapy did not improve the outcome in patients with stable coronary artery disease without clinical heart failure (no evidence of left ventricular systolic dysfunction, in the overall study population mean ejection fraction was 56.4%). In the subgroup analysis of the study, ivabradine was associated with an increase in the incidence of the primary end point (death from cardiovascular causes or nonfatal myocardial infarction) among patients who had angina of Canadian Cardiovascular Society class II or higher but not among patients without angina or those who had angina of class I. Ivabradine was associated with an increased incidence of bradycardia, QT prolongation, and atrial fibrillation.[99]Fox K, Ford I, Steg PG, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014 Sep 18;371(12):1091-9.
http://www.nejm.org/doi/full/10.1056/NEJMoa1406430#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25176136?tool=bestpractice.com
Digoxin significantly reduces the risk of composite end point of mortality or hospitalization in ambulatory chronic heart failure patients with NYHA class 3 or 4 symptoms, LVEF <25%, or cardiothoracic ratio of >55%, and should be considered in these patients.[100]Gheorghiade M, Patel K, Filippatos G, et al. Effect of oral digoxin in high-risk heart failure patients: a pre-specified subgroup analysis of the DIG trial. Eur J Heart Fail. 2013 May;15(5):551-9.
http://onlinelibrary.wiley.com/doi/10.1093/eurjhf/hft010/full
http://www.ncbi.nlm.nih.gov/pubmed/23355060?tool=bestpractice.com
In patients with heart failure who are in sinus rhythm, use of digoxin has no effect on mortality but is associated with a lower rate of hospitalization and clinical deterioration.[101]Hood WB Jr, Dans AL, Guyatt GH, et al. Digitalis for treatment of heart failure in patients in sinus rhythm. Cochrane Database Syst Rev. 2014 Apr 28;(4):CD002901.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002901.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/24771511?tool=bestpractice.com
 ]
An angiotensin-II receptor antagonist/neprilysin inhibitor is recommended both as a first-line treatment for patients newly diagnosed with acute heart failure, and to replace ACE inhibitor (or angiotensin-II receptor antagonist) therapy in patients with chronic heart failure with reduced ejection fraction that remains symptomatic despite existing therapy.[2] The dose of these agents should be increased to the maximum tolerated dose depending upon BP and heart rate. Typically, beta-blockers are started only after patients have stabilized, but should continue long term to reduce the risk of major cardiovascular events even if symptoms do not improve.[2]
]
An angiotensin-II receptor antagonist/neprilysin inhibitor is recommended both as a first-line treatment for patients newly diagnosed with acute heart failure, and to replace ACE inhibitor (or angiotensin-II receptor antagonist) therapy in patients with chronic heart failure with reduced ejection fraction that remains symptomatic despite existing therapy.[2] The dose of these agents should be increased to the maximum tolerated dose depending upon BP and heart rate. Typically, beta-blockers are started only after patients have stabilized, but should continue long term to reduce the risk of major cardiovascular events even if symptoms do not improve.[2]