Hypernatremia
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
free water losses
oral or intravenous fluids
Typically seen in acutely ill patients with conditions such as diarrhea and vomiting, poorly controlled diabetes mellitus, or during recovery from renal failure or obstructive uropathy.
The basic treatment strategy for all patients involves the following steps:
1. Calculating the free water deficit
2. Determining a suitable serum sodium correction rate
3. Estimating ongoing free water losses (if applicable)
4. Designing a suitable fluid repletion program that takes into account the estimated free water deficit, the desired serum sodium correction rate, and any ongoing free water losses.
Fluid quantity: calculating the free water deficit is a good starting point to determine how much fluid to give initially in order to correct the abnormal serum sodium concentration. The free water deficit does not take any ongoing fluid losses into account.
[Figure caption and citation for the preceding image starts]: Free water deficit formula. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). Na = sodiumCreated by the BMJ Knowledge Centre [Citation ends].
For example, an older male patient who weighs 70 kg and has a serum sodium concentration of 155 mEq/L would require 3.8 L of fluid to return his serum sodium concentration to a level of 140 mEq/L (i.e., [0.5 × 70] × [155-140/140]).
The Adrogué-Madias formula is often used in place of the free water deficit formula, as it takes into account the effect of specific fluid intake on the serum sodium concentration. It allows a prediction in the change in serum sodium concentration after the infusion of 1 L of an intravenous fluid of known sodium concentration.[Figure caption and citation for the preceding image starts]: Adrogué-Madias formula. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). Na = sodium. Sodium concentration of common fluids (per liter): normal saline (0.9%) - 154 mEq/L; lactated Ringer solution - 130 mEq/L; half-normal saline (0.45%) - 77 mEq/L; dextrose 5% in water - 0 mEq/L; enteral water - 0 mEq/LCreated by the BMJ Knowledge Centre [Citation ends].
The Adrogue-Madias formula does not take the ongoing water and electrolyte losses through urine and stool into account.[46]Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000 May 18;342(20):1493-9. http://www.ncbi.nlm.nih.gov/pubmed/10816188?tool=bestpractice.com In addition, it is only accurate if only one liter of fluid is infused. An adapted version of the Adrogue-Madias formula, developed in 2020, can be used to determine the volume of fluid required to change the serum sodium concentration by a specific amount. [83]Chen S, Shieh M, Chiaramonte R, et al. Improving on the Adrogué-Madias formula. Kidney360. 2021 Feb 25;2(2):365-70. https://journals.lww.com/kidney360/Fulltext/2021/02000/Improving_on_the_Adrogu__Madias_Formula.22.aspx http://www.ncbi.nlm.nih.gov/pubmed/35373033?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: V = volume needed. Na = sodium. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). K = potassium. [Na]2 = the desired change in sodium concentrationCreated by the BMJ Knowledge Centre [Citation ends].
The volume calculated from this should be added to the amount of water lost via electrolyte-free water excretion during the treatment period.[Figure caption and citation for the preceding image starts]: Electrolyte-free water excretion formula. V = urine flow rate. UNa = urine concentration of sodium (mEq/L). UK = urine concentration of potassium (mEq/L). PNa = plasma concentration of sodium (mEq/L)Created by the BMJ Knowledge Centre [Citation ends].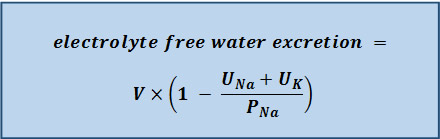
Serum sodium correction rate: patients with severe symptoms (i.e., neurologic symptoms) require more urgent treatment and more rapid correction of the sodium level for the first 2-3 hours to prevent long-term neurological complications (e.g., myelinolysis). In the first few hours, the serum sodium concentration should be lowered by 2 mEq/L/hour, followed by a correction rate of around 0.5 mEq/L/hour.[66]Sterns RH. Disorders of plasma sodium - causes, consequences, and correction. N Engl J Med. 2015 Jan 1;372(1):55-65. http://www.ncbi.nlm.nih.gov/pubmed/25551526?tool=bestpractice.com The acute hypernatremia correction rate should be based on careful monitoring of the symptoms/signs, volume status, serum sodium concentration, urine osmolality/electrolytes, and urine output. The aim is to lower the serum sodium level by 10 mEq/L/day in these patients if possible.[46]Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000 May 18;342(20):1493-9. http://www.ncbi.nlm.nih.gov/pubmed/10816188?tool=bestpractice.com If the hypernatremia has developed rapidly, theoretically there is not a concern about brain edema with normalization of the elevated serum sodium.[66]Sterns RH. Disorders of plasma sodium - causes, consequences, and correction. N Engl J Med. 2015 Jan 1;372(1):55-65. http://www.ncbi.nlm.nih.gov/pubmed/25551526?tool=bestpractice.com However, actual data regarding the results of different treatment approaches are lacking.
A correction rate of 0.5 mEq/L/hour is commonly used in patients with chronic hypernatremia.[1]Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015 Mar 1;91(5):299-307. https://www.aafp.org/afp/2015/0301/p299.html http://www.ncbi.nlm.nih.gov/pubmed/25822386?tool=bestpractice.com Current thinking is that sodium balance in these patients should be corrected slowly, as it is likely that the hypernatremia has developed over a long period of time, and the brain cells have had time to adapt to the high serum sodium concentration and elevated serum osmolality. Some studies have found that a more rapid correction (e.g., 3 days) may be desirable.[81]Bataille S, Baralla C, Torro D, et al. Undercorrection of hypernatremia is frequent and associated with mortality. BMC Nephrol. 2014 Feb 21;15:37. https://bmcnephrol.biomedcentral.com/articles/10.1186/1471-2369-15-37 http://www.ncbi.nlm.nih.gov/pubmed/24559470?tool=bestpractice.com [88]Heydarian F, Rezaeian A. Relationship between changes in serum sodium level and seizures occurrence in children with hypernatremic dehydration. Iran J Child Neurol. 2013 Fall;7(4):35-40. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3943043 http://www.ncbi.nlm.nih.gov/pubmed/24665316?tool=bestpractice.com [89]Darmon M, Pichon M, Schwebel C, et al. Influence of early dysnatremia correction on survival of critically ill patients. Shock. 2014 May;41(5):394-9. http://www.ncbi.nlm.nih.gov/pubmed/24667611?tool=bestpractice.com However, this approach is by no means definitive.
The rate of correction in children should not exceed 0.5 mEq/L/hour due to the high risk of seizures.[55]Berl T, Schrier RW. Disorders of water homeostatis. In: Schrier RW, 7th ed. Renal and electrolyte disorders. Philadelphia, PA: Lippincott, Williams and Wilkins; 2010:1-44.
Ongoing free water losses: replacing the free water deficit will usually correct the sodium abnormality in patients with hypovolemic hypernatremia.
In patients with free water losses and high electrolyte-free water excretion, replacing the free water deficit may fail to normalize the abnormal serum sodium concentration. Therefore, ongoing water losses also need to be taken into account.
In patients with ongoing urinary losses, the electrolyte-free water excretion should be calculated soon as possible.[Figure caption and citation for the preceding image starts]: Electrolyte-free water excretion formula. V = urine flow rate. UNa = urine concentration of sodium (mEq/L). UK = urine concentration of potassium (mEq/L). PNa = plasma concentration of sodium (mEq/L)Created by the BMJ Knowledge Centre [Citation ends].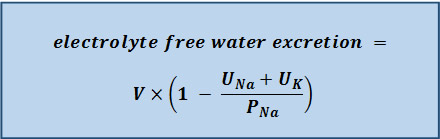
The calculated amount of water should be administered in order to just maintain (or steady) the serum sodium concentration at the level from the preceding day (if a 24-hour urine sample is collected for the electrolyte-free water excretion measurement), so that the serum sodium concentration will not increase further. If urine output or the electrolyte content of the urine changes, then the electrolyte-free water excretion should be recalculated. Free water losses can also occur in patients with hypernatremia associated with inadequate free water intake or excess sodium intake; however, they are usually minimal, and replacing ongoing water losses in these patients is likely to make little, or no, difference as the electrolyte-free water excretion is usually very low.
Fluid repletion program: the initial fluid repletion program should be based on calculating the free water deficit and the desired serum sodium correction rate.
In patients with hypernatremia associated with free water losses, the amount of fluid that is actually needed to correct the abnormal serum sodium concentration is determined by adding the water deficit amount to the amount of water lost via electrolyte-free water excretion during the treatment period.
If the patient is hypovolemic and in shock, the intravascular volume should be restored urgently, usually with normal saline (0.9%), prior to free water replacement.
The initial infusion rate depends on the patient's urine output and electrolyte-free water excretion. Some physicians recommend an initial rate of approximately 3-6 mL/kg/hour (acute hypernatremia) or 1.35 mL/kg/hour (chronic hypernatremia); however, the rate of administration should be adjusted based on signs/symptoms and laboratory data.
Overall, it is common practice for half of the free water deficit to be given in the first 24 hours, with the remaining half given in the next 24 hours.[55]Berl T, Schrier RW. Disorders of water homeostatis. In: Schrier RW, 7th ed. Renal and electrolyte disorders. Philadelphia, PA: Lippincott, Williams and Wilkins; 2010:1-44. For example, if the total water deficit is 3.8 L, this would mean giving approximately 1.9 L of fluid per day (i.e., approximately 80 mL/hour).
Replacing free water enterally is preferred when possible. If the patient is unable to take water orally, administration via a nasogastric tube is recommended.
If enteral intake is not possible, intravenous administration is required. Fluid choices include dextrose 5% in water, balanced solutions such as lactated Ringer solution, and saline (e.g., 0.45% or 0.90%). Dextrose is preferred in most patients, while saline is generally reserved for patients with signs of severe volume depletion such as hypotension. Intravenous fluids containing sodium (which includes saline and lactated Ringer solution) should not be administered to patients with hypernatremia associated with free water losses (unless they are severely hypotensive or in shock) or accidental or iatrogenic excess intake of sodium.
In patients infused with dextrose 5% in water, the patient should be monitored for the development of hyperglycemia and associated dextrose-induced osmotic diuresis, which can worsen the hypernatremia. If hypernatremia is worsened by the patient's inability to metabolize the glucose load, a less concentrated solution of dextrose (e.g., dextrose 2.5% in water), or even pure water, may be given.
Co-existing electrolyte abnormalities (e.g., hypokalemia) should be corrected.
The regimen should always be adjusted according to patient response and serum sodium levels, rather than relying on amounts calculated from formulas.
treat underlying cause
Treatment recommended for ALL patients in selected patient group
Treatment of the underlying cause (e.g., giving insulin, treating renal failure/obstructive uropathy, treating nausea/diarrhea/fever) should be a priority.
Causative medications (e.g., mannitol, loop diuretics, activated charcoal/sorbitol) should be ceased.
monitoring
Treatment recommended for ALL patients in selected patient group
Monitoring is especially important during the first few hours of correction in symptomatic or rapidly developing (acute) hypernatremia.
Frequent measurement of the serum sodium concentration (e.g., every 1-2 hours for acute hypernatremia or every 4-6 hours for chronic hypernatremia until stable, then every 12-24 hours) is necessary to make sure that levels are returning to the correct range at the desired rate.
Other parameters that should be monitored throughout the treatment period include urine sodium concentration, urine osmolality, and urine output. This information helps determine the amount of water needed to replace the ongoing losses.
Serum electrolytes should also be monitored to assess for electrolyte imbalances (e.g., hypokalemia), and serum glucose to assess for treatment-related hyperglycemia (if dextrose-containing solutions are used).
desmopressin (if central diabetes insipidus)
Treatment recommended for SOME patients in selected patient group
Treatment of choice in patients with central diabetes insipidus is desmopressin (DDAVP), a synthetic analog of vasopressin (also known as arginine vasopressin [AVP] or antidiuretic hormone [ADH]).
Desmopressin reduces urinary losses (and electrolyte-free water excretion), and can be administered orally, intranasally, or parenterally.[90]Baldeweg SE, Ball S, Brooke A, et al. Society for Endocrinology clinical guidance: inpatient management of cranial diabetes insipidus. Endor Connect. 2018 Jul;7 (7):G8-11. https://ec.bioscientifica.com/view/journals/ec/7/7/EC-18-0154.xml http://www.ncbi.nlm.nih.gov/pubmed/29930026?tool=bestpractice.com
Treatment should be started with a low dose, which is increased gradually according to response.
Desmopressin therapy is usually sufficient to stop ongoing water losses, though previous losses do need to be replaced.
Desmopressin is not effective in nephrogenic diabetes insipidus.
Hypernatremia in the setting of central diabetes insipidus should be treated and managed as a medical emergency in a high-dependency area. In patients who are intravascularly depleted, intravenous rehydration with 0.9% sodium chloride should precede consideration of free water administration.[90]Baldeweg SE, Ball S, Brooke A, et al. Society for Endocrinology clinical guidance: inpatient management of cranial diabetes insipidus. Endor Connect. 2018 Jul;7 (7):G8-11. https://ec.bioscientifica.com/view/journals/ec/7/7/EC-18-0154.xml http://www.ncbi.nlm.nih.gov/pubmed/29930026?tool=bestpractice.com
See Diabetes insipidus.
Primary options
desmopressin: children: consult specialist for guidance on dose; adults: 0.1 to 1.2 mg/day orally given in 2-3 divided doses; 10-40 micrograms/day intranasally given in 1-3 divided doses; 2-4 micrograms/day intravenously given in 2 divided doses
thiazide diuretic (if nephrogenic diabetes insipidus)
Treatment recommended for SOME patients in selected patient group
Patients with nephrogenic diabetes insipidus do not respond to desmopressin and will, therefore, require large amounts of water just to keep their serum sodium concentration the same, and even more water to correct the hypernatremia. The route of administration depends on the clinical status of the patient. If they have altered mental status, the intravenous route is preferred. If they are relatively well and able to drink water, the oral route is preferred. Adding the electrolyte-free water excretion to the free water deficit will help in working out a starting point of how much water to give.
Thiazide diuretics interfere with the diluting ability of the kidney and cause mild volume depletion and increased proximal reabsorption of sodium and water. As such, they are helpful in decreasing the urine output in patients with nephrogenic diabetes insipidus.
See Diabetes insipidus.
Primary options
hydrochlorothiazide: children: 1-3 mg/kg/day orally given in 2 divided doses; adults: 25-50 mg orally once daily
renal replacement therapy
Recommended in patients with concomitant renal failure or fluid overload.
With continuous slow administration, and adjustments of the sodium level in the dialysate or in the replacement fluid, continuous renal replacement therapies have resulted in good outcomes.[91]Dangoisse C, Dickie H, Tovey L, et al. Correction of hyper- and hyponatraemia during continuous renal replacement therapy. Nephron Clin Pract. 2014;128(3-4):394-8. http://www.ncbi.nlm.nih.gov/pubmed/25592652?tool=bestpractice.com [92]Medow JE, Sanghvi SR, Hofmann RM. Use of high-flow continuous renal replacement therapy with citrate anticoagulation to control intracranial pressure by maintaining hypernatremia in a patient with acute brain injury and renal failure. Clin Med Res. 2015 Jun;13(2):89-93. http://www.clinmedres.org/content/13/2/89.full http://www.ncbi.nlm.nih.gov/pubmed/25487240?tool=bestpractice.com [93]Giabicani M, Guitard PG, Guerrot D, et al. Successful treatment of extreme hypernatremia by continuous veno-venous hemodiafiltration [in French]. Nephrol Ther. 2015 Nov;11(6):492-5. http://www.ncbi.nlm.nih.gov/pubmed/26169976?tool=bestpractice.com [94]Korvenius Jørgensen H, Haug AC, Gilsaa T. Severe hypernatraemia can be treated with continuous veno-venous haemodialysis [in Danish]. Ugeskr Laeger. 2013 Sep 23;175(39):2255-6. http://www.ncbi.nlm.nih.gov/pubmed/24063713?tool=bestpractice.com [95]Pazmiño PA, Pazmiño BP. Treatment of acute hypernatremia with hemodialysis. Am J Nephrol. 1993;13(4):260-5. http://www.ncbi.nlm.nih.gov/pubmed/8267023?tool=bestpractice.com [96]Choi JH, Lee HS, Kim SM, et al. Paranoid adipsia-induced severe hypernatremia and uremia treated with hemodialysis. Electrolyte Blood Press. 2013 Jun;11(1):29-32. https://synapse.koreamed.org/DOIx.php?id=10.5049/EBP.2013.11.1.29 http://www.ncbi.nlm.nih.gov/pubmed/23946763?tool=bestpractice.com [97]Park HS, Hong YA, Kim HG, et al. Usefulness of continuous renal replacement therapy for correcting hypernatremia in a patient with severe congestive heart failure. Hemodial Int. 2012 Oct;16(4):559-63. http://www.ncbi.nlm.nih.gov/pubmed/22515501?tool=bestpractice.com [98]Yang CW, Kim YS, Park IS, et al. Treatment of severe acute hypernatremia and renal failure by hemodialysis. Nephron. 1995;70(3):372-3. http://www.ncbi.nlm.nih.gov/pubmed/7477631?tool=bestpractice.com [99]Yang YF, Wu VC, Huang CC. Successful management of extreme hypernatraemia by haemofiltration in a patient with severe metabolic acidosis and renal failure. Nephrol Dial Transplant. 2005 Sep;20(9):2013-4. https://academic.oup.com/ndt/article/20/9/2013/1850324 http://www.ncbi.nlm.nih.gov/pubmed/15985507?tool=bestpractice.com Use of slow dialysis, in the form of continuous dialysis or sustained low-efficiency dialysis, has only been reported in patients with acute hypernatremia.
Hemodialysis and hemofiltration may correct the sodium level too quickly. One study found that correcting the serum sodium concentration by >1 mEq/L/hour in acute severe hypernatremia was associated with higher mortality.[100]Ma F, Liu Y, Bai M, et al. The reduction rate of serum sodium and mortality in patients undergoing continuous venovenous hemofiltration for acute severe hypernatremia. Am J Med Sci. 2016 Jun 11;352(3):272-9. http://www.ncbi.nlm.nih.gov/pubmed/27650232?tool=bestpractice.com Adverse outcomes may be avoided by appropriate increases in the sodium level of the dialysate or replacement fluid, and reduction of the rate of correction by using slower blood and dialysate flows and lower-efficiency dialyzers (e.g., sustained low-efficiency dialysis).
Peritoneal dialysis using a low-sodium dialysate has been found to be effective in treating hypernatremia due to renal failure.[101]Moritz ML, del Rio M, Crooke GA, et al. Acute peritoneal dialysis as both cause and treatment of hypernatremia in an infant. Pediatr Nephrol. 2001 Sep;16(9):697-700. http://www.ncbi.nlm.nih.gov/pubmed/11511979?tool=bestpractice.com
inadequate free water intake
oral or intravenous fluids
Typically occurs in a nursing home resident with dementia. Hypernatremia is relatively easy to correct in these patients by giving more free water. However, if the patient is hypovolemic and in shock, the intravascular volume should be restored urgently, usually with normal saline (0.9%), prior to free water replacement.
Once intravascular volume has been urgently restored, the basic treatment strategy for all patients involves the following steps:
1. Calculating the free water deficit
2. Determining a suitable serum sodium correction rate
3. Estimating ongoing free water losses (if applicable)
4. Designing a suitable fluid repletion program that takes into account the estimated free water deficit, the desired serum sodium correction rate, and any ongoing free water losses.
Fluid quantity: calculating the free water deficit is a good starting point to determine how much fluid to give initially in order to correct the abnormal serum sodium concentration. The free water deficit does not take any ongoing fluid losses into account.[Figure caption and citation for the preceding image starts]: Free water deficit formula. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). Na = sodiumCreated by the BMJ Knowledge Centre [Citation ends].
For example, an older male patient who weighs 70 kg and has a serum sodium concentration of 155 mEq/L would require 3.8 L of fluid to return his serum sodium concentration to a level of 140 mEq/L (i.e., [0.5 × 70] × [155-140/140]).
The Adrogué-Madias formula is often used in place of the free water deficit formula, as it takes into account the effect of specific fluid intake on the serum sodium concentration. It allows a prediction in the change in serum sodium concentration after the infusion of 1 L of an intravenous fluid of known sodium concentration.[Figure caption and citation for the preceding image starts]: Adrogué-Madias formula. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). Na = sodium. Sodium concentration of common fluids (per liter): normal saline (0.9%) - 154 mEq/L; lactated Ringer solution - 130 mEq/L; half-normal saline (0.45%) - 77 mEq/L; dextrose 5% in water - 0 mEq/L; enteral water - 0 mEq/LCreated by the BMJ Knowledge Centre [Citation ends].
The Adrogue-Madias formula does not take the ongoing water and electrolyte losses through urine and stool into account.[46]Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000 May 18;342(20):1493-9. http://www.ncbi.nlm.nih.gov/pubmed/10816188?tool=bestpractice.com In addition, it is only accurate if only one liter of fluid is infused. An adapted version of the Adrogue-Madias formula, developed in 2020, can be used to determine the volume of fluid required to change the serum sodium concentration by a specific amount. [83]Chen S, Shieh M, Chiaramonte R, et al. Improving on the Adrogué-Madias formula. Kidney360. 2021 Feb 25;2(2):365-70. https://journals.lww.com/kidney360/Fulltext/2021/02000/Improving_on_the_Adrogu__Madias_Formula.22.aspx http://www.ncbi.nlm.nih.gov/pubmed/35373033?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: V = volume needed. Na = sodium. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). K = potassium. [Na]2 = the desired change in sodium concentrationCreated by the BMJ Knowledge Centre [Citation ends].
The volume calculated from this formula should be added to the amount of water lost via electrolyte-free water excretion during the treatment period.[Figure caption and citation for the preceding image starts]: Electrolyte-free water excretion formula. V = urine flow rate. UNa = urine concentration of sodium (mEq/L). UK = urine concentration of potassium (mEq/L). PNa = plasma concentration of sodium (mEq/L)Created by the BMJ Knowledge Centre [Citation ends].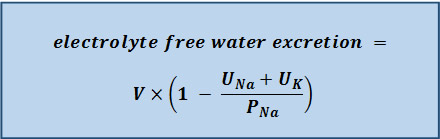
Serum sodium correction rate: patients with severe symptoms (i.e., neurologic symptoms) require more urgent treatment and more rapid correction of the sodium level for the first 2-3 hours to prevent long-term neurologic complications (e.g., myelinolysis). In the first few hours, the serum sodium concentration should be lowered by 2 mEq/L/hour, followed by a correction rate of around 0.5 mEq/L/hour.[66]Sterns RH. Disorders of plasma sodium - causes, consequences, and correction. N Engl J Med. 2015 Jan 1;372(1):55-65. http://www.ncbi.nlm.nih.gov/pubmed/25551526?tool=bestpractice.com The acute hypernatremia correction rate should be based on careful monitoring of the symptoms/signs, volume status, serum sodium concentration, urine osmolality/electrolytes, and urine output. The aim is to lower the serum sodium level by 10 mEq/L/day in these patients if possible.[46]Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000 May 18;342(20):1493-9. http://www.ncbi.nlm.nih.gov/pubmed/10816188?tool=bestpractice.com If the hypernatremia has developed rapidly, theoretically there is not a concern about brain edema with normalization of the elevated serum sodium.[66]Sterns RH. Disorders of plasma sodium - causes, consequences, and correction. N Engl J Med. 2015 Jan 1;372(1):55-65. http://www.ncbi.nlm.nih.gov/pubmed/25551526?tool=bestpractice.com However, actual data regarding the results of different treatment approaches are lacking. A correction rate of 0.5 mEq/L/hour is commonly used in patients with chronic hypernatremia.[1]Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015 Mar 1;91(5):299-307. https://www.aafp.org/afp/2015/0301/p299.html http://www.ncbi.nlm.nih.gov/pubmed/25822386?tool=bestpractice.com
Current thinking is that sodium balance in these patients should be corrected slowly, as it is likely that the hypernatremia has developed over a long period of time, and the brain cells have had time to adapt to the high serum sodium concentration and elevated serum osmolality. Some studies have found that a more rapid correction (e.g., 3 days) may be desirable.[81]Bataille S, Baralla C, Torro D, et al. Undercorrection of hypernatremia is frequent and associated with mortality. BMC Nephrol. 2014 Feb 21;15:37. https://bmcnephrol.biomedcentral.com/articles/10.1186/1471-2369-15-37 http://www.ncbi.nlm.nih.gov/pubmed/24559470?tool=bestpractice.com [88]Heydarian F, Rezaeian A. Relationship between changes in serum sodium level and seizures occurrence in children with hypernatremic dehydration. Iran J Child Neurol. 2013 Fall;7(4):35-40. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3943043 http://www.ncbi.nlm.nih.gov/pubmed/24665316?tool=bestpractice.com [89]Darmon M, Pichon M, Schwebel C, et al. Influence of early dysnatremia correction on survival of critically ill patients. Shock. 2014 May;41(5):394-9. http://www.ncbi.nlm.nih.gov/pubmed/24667611?tool=bestpractice.com However, this approach is by no means definitive.
The rate of correction in children should not exceed 0.5 mEq/L/hour due to the high risk of seizures.[55]Berl T, Schrier RW. Disorders of water homeostatis. In: Schrier RW, 7th ed. Renal and electrolyte disorders. Philadelphia, PA: Lippincott, Williams and Wilkins; 2010:1-44.
Ongoing free water losses: replacing the free water deficit will usually correct the sodium abnormality in patients with hypovolemic hypernatremia.
In patients with free water losses and high electrolyte-free water excretion, replacing the free water deficit may fail to normalize the abnormal serum sodium concentration. Therefore, ongoing water losses also need to be taken into account.
In patients with ongoing urinary losses, calculate the electrolyte-free water excretion soon as possible.[Figure caption and citation for the preceding image starts]: Electrolyte-free water excretion formula. V = urine flow rate. UNa = urine concentration of sodium (mEq/L). UK = urine concentration of potassium (mEq/L). PNa = plasma concentration of sodium (mEq/L)Created by the BMJ Knowledge Centre [Citation ends].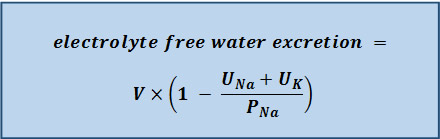
The calculated amount of water should be administered in order to just maintain (or steady) the serum sodium concentration at the level from the preceding day (if a 24-hour urine sample is collected for the electrolyte-free water excretion measurement), so that the serum sodium concentration will not increase further. If urine output or the electrolyte content of the urine changes, then the electrolyte-free water excretion should be recalculated.
Free water losses can also occur in patients with hypernatremia associated with inadequate free water intake or excess sodium intake; however, they are usually minimal, and replacing ongoing water losses in these patients is likely to make little, or no, difference as the electrolyte-free water excretion is usually very low.
Fluid repletion program: the initial fluid repletion program should be based on calculating the free water deficit and the desired serum sodium correction rate.
In patients with hypernatremia associated with free water losses, the amount of fluid that is actually needed to correct the abnormal serum sodium concentration is determined by adding the water deficit amount to the amount of water lost via electrolyte-free water excretion during the treatment period.
If the patient is hypovolemic and in shock, the intravascular volume should be restored urgently, usually with normal saline (0.9%), prior to free water replacement.
The initial infusion rate depends on the patient's urine output and electrolyte-free water excretion. Some physicians recommend an initial rate of approximately 3-6 mL/kg/hour (acute hypernatremia) or 1.35 mL/kg/hour (chronic hypernatremia); however, the rate of administration should be adjusted based on signs/symptoms and laboratory data.
Overall, it is common practice for half of the free water deficit to be given in the first 24 hours, with the remaining half given in the next 24 hours.[55]Berl T, Schrier RW. Disorders of water homeostatis. In: Schrier RW, 7th ed. Renal and electrolyte disorders. Philadelphia, PA: Lippincott, Williams and Wilkins; 2010:1-44. For example, if the total water deficit is 3.8 L, this would mean giving approximately 1.9 L of fluid per day (i.e., approximately 80 mL/hour).
Replacing free water enterally is preferred when possible. If the patient is unable to take water orally, administration via a nasogastric tube is recommended.
If enteral intake is not possible, intravenous administration is required. Fluid choices include dextrose 5% in water, balanced solutions such as lactated Ringer solution, and saline (e.g., 0.45% or 0.90%). Dextrose is preferred in most patients, while saline is generally reserved for patients with signs of severe volume depletion such as hypotension. Intravenous fluids containing sodium (which includes saline and lactated Ringer solution) should not be administered to patients with hypernatremia associated with free water losses (unless they are severely hypotensive or in shock) or accidental or iatrogenic excess intake of sodium.
In patients infused with dextrose 5% in water, the patient should be monitored for the development of hyperglycemia and associated dextrose-induced osmotic diuresis, which can worsen the hypernatremia. If hypernatremia is worsened by the patient's inability to metabolize the glucose load, a less concentrated solution of dextrose (e.g., dextrose 2.5% in water), or even pure water, may be given.
Co-existing electrolyte abnormalities (e.g., hypokalemia) should be corrected. The regimen should always be adjusted according to patient response and serum sodium levels, rather than relying on amounts calculated from formulas.
treat underlying cause
Treatment recommended for ALL patients in selected patient group
Treatment of the underlying cause (e.g., encouraging the patient to drink more water, treating primary hypodipsia) should be a priority.
monitoring
Treatment recommended for ALL patients in selected patient group
Monitoring is especially important during the first few hours of correction in symptomatic or rapidly developing (acute) hypernatremia.
Frequent measurement of the serum sodium concentration (e.g., every 1-2 hours for acute hypernatremia or every 4-6 hours for chronic hypernatremia until stable, then every 12-24 hours) is necessary to make sure that levels are returning to the correct range at the desired rate.
Other parameters that should be monitored throughout the treatment period include urine sodium concentration, urine osmolality, and urine output. This information helps determine the amount of water needed to replace the ongoing losses.
Serum electrolytes should also be monitored to assess for electrolyte imbalances (e.g., hypokalemia), and serum glucose to assess for treatment-related hyperglycemia (if dextrose-containing solutions are used).
accidental or iatrogenic excess intake of sodium
oral or intravenous fluids
As patients are not volume depleted, they should receive only enough free water to correct the hypernatremia.
The basic treatment strategy for all patients involves the following steps:
1. Calculating the free water deficit
2. Determining a suitable serum sodium correction rate
3. Estimating ongoing free water losses (if applicable)
4. Designing a suitable fluid repletion program that takes into account the estimated free water deficit, the desired serum sodium correction rate, and any ongoing free water losses.
Fluid quantity: calculating the free water deficit is a good starting point to determine how much fluid to give initially in order to correct the abnormal serum sodium concentration. The free water deficit does not take any ongoing fluid losses into account.[Figure caption and citation for the preceding image starts]: Free water deficit formula. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). Na = sodiumCreated by the BMJ Knowledge Centre [Citation ends].
For example, an older male patient who weighs 70 kg and has a serum sodium concentration of 155 mEq/L would require 3.8 L of fluid to return his serum sodium concentration to a level of 140 mEq/L (i.e., [0.5 × 70] × [155-140/140]).
The Adrogué-Madias formula is often used in place of the free water deficit formula, as it takes into account the effect of specific fluid intake on the serum sodium concentration. It allows a prediction in the change in serum sodium concentration after the infusion of 1 L of an intravenous fluid of known sodium concentration.[Figure caption and citation for the preceding image starts]: Adrogué-Madias formula. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). Na = sodium. Sodium concentration of common fluids (per liter): normal saline (0.9%) - 154 mEq/L; lactated Ringer solution - 130 mEq/L; half-normal saline (0.45%) - 77 mEq/L; dextrose 5% in water - 0 mEq/L; enteral water - 0 mEq/LCreated by the BMJ Knowledge Centre [Citation ends].
The Adrogue-Madias formula does not take the ongoing water and electrolyte losses through urine and stool into account.[46]Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000 May 18;342(20):1493-9. http://www.ncbi.nlm.nih.gov/pubmed/10816188?tool=bestpractice.com In addition, it is only accurate if only one liter of fluid is infused. An adapted version of the Adrogue-Madias formula, developed in 2020, can be used to determine the volume of fluid required to change the serum sodium concentration by a specific amount. [83]Chen S, Shieh M, Chiaramonte R, et al. Improving on the Adrogué-Madias formula. Kidney360. 2021 Feb 25;2(2):365-70. https://journals.lww.com/kidney360/Fulltext/2021/02000/Improving_on_the_Adrogu__Madias_Formula.22.aspx http://www.ncbi.nlm.nih.gov/pubmed/35373033?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: V = volume needed. Na = sodium. TBW (total body water) = patient body weight (kg) x 0.5 (women/older men) or 0.6 (young men or children) or 0.4 (dehydrated patients). K = potassium. [Na]2 = the desired change in sodium concentrationCreated by the BMJ Knowledge Centre [Citation ends].
The volume calculated from this formula should be added to the amount of water lost via electrolyte-free water excretion during the treatment period.[Figure caption and citation for the preceding image starts]: Electrolyte-free water excretion formula. V = urine flow rate. UNa = urine concentration of sodium (mEq/L). UK = urine concentration of potassium (mEq/L). PNa = plasma concentration of sodium (mEq/L)Created by the BMJ Knowledge Centre [Citation ends].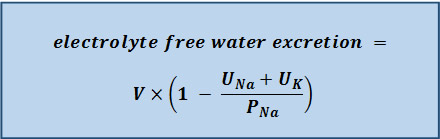
Serum sodium correction rate: patients with severe symptoms (i.e., neurologic symptoms) require more urgent treatment and more rapid correction of the sodium level for the first 2-3 hours to prevent long-term neurologic complications (e.g., myelinolysis). In the first few hours, the serum sodium concentration should be lowered by 2 mEq/L/hour, followed by a correction rate of around 0.5 mEq/L/hour.[66]Sterns RH. Disorders of plasma sodium - causes, consequences, and correction. N Engl J Med. 2015 Jan 1;372(1):55-65. http://www.ncbi.nlm.nih.gov/pubmed/25551526?tool=bestpractice.com The acute hypernatremia correction rate should be based on careful monitoring of the symptoms/signs, volume status, serum sodium concentration, urine osmolality/electrolytes, and urine output. The aim is to lower the serum sodium level by 10 mEq/L/day in these patients if possible.[46]Adrogué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000 May 18;342(20):1493-9. http://www.ncbi.nlm.nih.gov/pubmed/10816188?tool=bestpractice.com If the hypernatremia has developed rapidly, theoretically there is not a concern about brain edema with normalization of the elevated serum sodium.[66]Sterns RH. Disorders of plasma sodium - causes, consequences, and correction. N Engl J Med. 2015 Jan 1;372(1):55-65. http://www.ncbi.nlm.nih.gov/pubmed/25551526?tool=bestpractice.com However, actual data regarding the results of different treatment approaches are lacking.
A correction rate of 0.5 mEq/L/hour is commonly used in patients with chronic hypernatremia.[1]Braun MM, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015 Mar 1;91(5):299-307. https://www.aafp.org/afp/2015/0301/p299.html http://www.ncbi.nlm.nih.gov/pubmed/25822386?tool=bestpractice.com Current thinking is that sodium balance in these patients should be corrected slowly, as it is likely that the hypernatremia has developed over a long period of time, and the brain cells have had time to adapt to the high serum sodium concentration and elevated serum osmolality. Some studies have found that a more rapid correction (e.g., 3 days) may be desirable.[81]Bataille S, Baralla C, Torro D, et al. Undercorrection of hypernatremia is frequent and associated with mortality. BMC Nephrol. 2014 Feb 21;15:37. https://bmcnephrol.biomedcentral.com/articles/10.1186/1471-2369-15-37 http://www.ncbi.nlm.nih.gov/pubmed/24559470?tool=bestpractice.com [88]Heydarian F, Rezaeian A. Relationship between changes in serum sodium level and seizures occurrence in children with hypernatremic dehydration. Iran J Child Neurol. 2013 Fall;7(4):35-40. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3943043 http://www.ncbi.nlm.nih.gov/pubmed/24665316?tool=bestpractice.com [89]Darmon M, Pichon M, Schwebel C, et al. Influence of early dysnatremia correction on survival of critically ill patients. Shock. 2014 May;41(5):394-9. http://www.ncbi.nlm.nih.gov/pubmed/24667611?tool=bestpractice.com However, this approach is by no means definitive.
The rate of correction in children should not exceed 0.5 mEq/L/hour due to the high risk of seizures.[55]Berl T, Schrier RW. Disorders of water homeostatis. In: Schrier RW, 7th ed. Renal and electrolyte disorders. Philadelphia, PA: Lippincott, Williams and Wilkins; 2010:1-44.
Ongoing free water losses: replacing the free water deficit will usually correct the sodium abnormality in patients with hypovolemic hypernatremia.
In patients with free water losses and high electrolyte-free water excretion, replacing the free water deficit may fail to normalize the abnormal serum sodium concentration. Therefore, ongoing water losses may also need to be taken into account.
In patients with ongoing urinary losses, calculate the electrolyte-free water excretion soon as possible.[Figure caption and citation for the preceding image starts]: Electrolyte-free water excretion formula. V = urine flow rate. UNa = urine concentration of sodium (mEq/L). UK = urine concentration of potassium (mEq/L). PNa = plasma concentration of sodium (mEq/L)Created by the BMJ Knowledge Centre [Citation ends].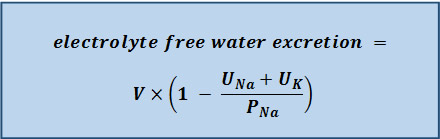
The calculated amount of water should be administered in order to just maintain (or steady) the serum sodium concentration at the level from the preceding day (if a 24-hour urine sample is collected for the electrolyte-free water excretion measurement), so that the serum sodium concentration will not increase further. If urine output or the electrolyte content of the urine changes, then the electrolyte-free water excretion should be recalculated.
Free water losses can also occur in patients with hypernatremia associated with inadequate free water intake or excess sodium intake; however, they are usually minimal, and replacing ongoing water losses in these patients is likely to make little, or no difference as the electrolyte-free water excretion is usually very low.
Fluid repletion program: the initial fluid repletion program should be based on calculating the free water deficit and the desired serum sodium correction rate.
In patients with hypernatremia associated with free water losses, the amount of fluid that is actually needed to correct the abnormal serum sodium concentration is determined by adding the water deficit amount to the amount of water lost via electrolyte-free water excretion during the treatment period.
If the patient is hypovolemic and in shock, the intravascular volume should be restored urgently, usually with normal saline (0.9%), prior to free water replacement.
The initial infusion rate depends on the patient's urine output and electrolyte-free water excretion. Some physicians recommend an initial rate of approximately 3-6 mL/kg/hour (acute hypernatremia) or 1.35 mL/kg/hour (chronic hypernatremia); however, the rate of administration should be adjusted based on signs/symptoms and laboratory data.
Overall, it is common practice for half of the free water deficit to be given in the first 24 hours, with the remaining half given in the next 24 hours.[55]Berl T, Schrier RW. Disorders of water homeostatis. In: Schrier RW, 7th ed. Renal and electrolyte disorders. Philadelphia, PA: Lippincott, Williams and Wilkins; 2010:1-44. For example, if the total water deficit is 3.8 L, this would mean giving approximately 1.9 L of fluid per day (i.e., approximately 80 mL/hour).
Replacing free water enterally is preferred when possible. If the patient is unable to take water orally, administration via a nasogastric tube is recommended.
If enteral intake is not possible, intravenous administration is required. Fluid choices include dextrose 5% in water, balanced solutions such as lactated Ringer solution, and saline (e.g., 0.45% or 0.90%). Dextrose is preferred in most patients, while saline is generally reserved for patients with signs of severe volume depletion such as hypotension. Intravenous fluids containing sodium (which includes saline and lactated Ringer solution) should not be administered to patients with hypernatremia associated with free water losses (unless they are severely hypotensive or in shock) or accidental or iatrogenic excess intake of sodium.
In patients infused with dextrose 5% in water, the patient should be monitored for the development of hyperglycemia and associated dextrose-induced osmotic diuresis, which can worsen the hypernatremia. If hypernatremia is worsened by the patient's inability to metabolize the glucose load, a less concentrated solution of dextrose (e.g., dextrose 2.5% in water), or even pure water, may be given.
Co-existing electrolyte abnormalities (e.g., hypokalemia) should be corrected.
The regimen should always be adjusted according to patient response and serum sodium levels, rather than relying on amounts calculated from formulas.
treat underlying cause
Treatment recommended for ALL patients in selected patient group
Any excess salt intake should be stopped immediately.
Typically caused by administration of multiple bolus doses of hypertonic sodium bicarbonate (e.g., in a patient with metabolic acidosis) or, much less commonly, by ingestion of large amounts of salt (e.g., deliberate ingestion of household-strength bleach [sodium hypochlorite], inadvertent infusion with 5% saline [rather than 5% dextrose], sea water drowning [in survivors thereof], inappropriately high concentration of sodium bicarbonate or sodium chloride in dialysate for hemodialysis treatment, and ingestion of bamboo salt [sea salt roasted in bamboo tubes], massive intake of seasoning soy sauce or general excessive salt ingestion [usually occurs in pediatric patients when salt may be mistaken for sugar]).[2]Sam R, Ing TS. Sodium and water disturbances. In: Lai KN, ed. A practical manual of renal medicine, nephrology, dialysis and transplantation. Singapore: World Scientific Publishing C.; 2009:45-79.[34]Ward MJ, Routledge PA. Hypernatraemia and hyperchloraemic acidosis after bleach ingestion. Hum Toxicol. 1988 Jan;7(1):37-8. http://www.ncbi.nlm.nih.gov/pubmed/3346039?tool=bestpractice.com [35]Ju HJ, Bae HJ, Choi DE, et al. Severe hypernatremia by excessive bamboo salt ingestion in healthy young woman. Electrolyte Blood Press. 2013 Dec;11(2):53-5. https://synapse.koreamed.org/DOIx.php?id=10.5049/EBP.2013.11.2.53 http://www.ncbi.nlm.nih.gov/pubmed/24627705?tool=bestpractice.com [36]Cassorla FG, Gill JR Jr, Gold PW, et al. Nosocomial hypernatremia. N Engl J Med. 1985 Aug 1;313(5):329. http://www.ncbi.nlm.nih.gov/pubmed/4010746?tool=bestpractice.com [37]Bhosale GP, Shah VR. Successful recovery from iatrogenic severe hypernatremia and severe metabolic acidosis resulting from accidental use of inappropriate bicarbonate concentrate for hemodialysis treatment. Saudi J Kidney Dis Transpl. 2015 Jan;26(1):107-10. http://www.ncbi.nlm.nih.gov/pubmed/25579726?tool=bestpractice.com [38]Hubert G, Liet JM, Barrière F, et al. Severe hypernatremia due to sea water ingestion in a child [in French]. Arch Pediatr. 2015 Jan;22(1):39-42. http://www.ncbi.nlm.nih.gov/pubmed/25282459?tool=bestpractice.com [39]Sakamoto A, Hoshino T, Boku K, et al. Fatal acute hypernatremia resulting from a massive intake of seasoning soy sauce. Acute Med Surg. 2020 Jan-Dec;7(1):e555. https://onlinelibrary.wiley.com/doi/10.1002/ams2.555 http://www.ncbi.nlm.nih.gov/pubmed/32832094?tool=bestpractice.com
monitoring
Treatment recommended for ALL patients in selected patient group
Monitoring is especially important during the first few hours of correction in symptomatic or rapidly developing (acute) hypernatremia.
Frequent measurement of the serum sodium concentration (e.g., every 1-2 hours for acute hypernatremia or every 4-6 hours for chronic hypernatremia until stable, then every 12-24 hours) is necessary to make sure that levels are returning to the correct range at the desired rate.
Other parameters that should be monitored throughout the treatment period include urine sodium concentration, urine osmolality, and urine output. This information helps determine the amount of water needed to replace the ongoing losses.
Serum electrolytes should also be monitored to assess for electrolyte imbalances (e.g., hypokalemia), and serum glucose to assess for treatment-related hyperglycemia (if dextrose-containing solutions are used).
loop diuretic
Treatment recommended for SOME patients in selected patient group
May be used in patients with hypernatremia associated with excess salt intake and fluid overload to increase renal sodium excretion; however, fluid losses during therapy should be replaced with free water. Loop diuretics do not correct the hypernatremia (if anything, they can make it worse); they are used to decrease the sodium overload in these patients.
Primary options
furosemide: children: 1 mg/kg intravenously initially, increase by 1 mg/kg/dose increments every 2 hours until response, then every 6-8 hours thereafter, maximum 600 mg/day; adults: 20-40 mg intravenously initially, increase by 20 mg/dose increments every 2 hours until response, then every 6-12 hours thereafter, maximum 600 mg/day
renal replacement therapy
May be used in patients with pre-existing advanced renal failure or those with fluid overload or sodium overload.
With continuous slow administration, and adjustments of the sodium level in the dialysate or in the replacement fluid, continuous renal replacement therapies have resulted in good outcomes.[91]Dangoisse C, Dickie H, Tovey L, et al. Correction of hyper- and hyponatraemia during continuous renal replacement therapy. Nephron Clin Pract. 2014;128(3-4):394-8. http://www.ncbi.nlm.nih.gov/pubmed/25592652?tool=bestpractice.com [92]Medow JE, Sanghvi SR, Hofmann RM. Use of high-flow continuous renal replacement therapy with citrate anticoagulation to control intracranial pressure by maintaining hypernatremia in a patient with acute brain injury and renal failure. Clin Med Res. 2015 Jun;13(2):89-93. http://www.clinmedres.org/content/13/2/89.full http://www.ncbi.nlm.nih.gov/pubmed/25487240?tool=bestpractice.com [93]Giabicani M, Guitard PG, Guerrot D, et al. Successful treatment of extreme hypernatremia by continuous veno-venous hemodiafiltration [in French]. Nephrol Ther. 2015 Nov;11(6):492-5. http://www.ncbi.nlm.nih.gov/pubmed/26169976?tool=bestpractice.com [94]Korvenius Jørgensen H, Haug AC, Gilsaa T. Severe hypernatraemia can be treated with continuous veno-venous haemodialysis [in Danish]. Ugeskr Laeger. 2013 Sep 23;175(39):2255-6. http://www.ncbi.nlm.nih.gov/pubmed/24063713?tool=bestpractice.com [95]Pazmiño PA, Pazmiño BP. Treatment of acute hypernatremia with hemodialysis. Am J Nephrol. 1993;13(4):260-5. http://www.ncbi.nlm.nih.gov/pubmed/8267023?tool=bestpractice.com [96]Choi JH, Lee HS, Kim SM, et al. Paranoid adipsia-induced severe hypernatremia and uremia treated with hemodialysis. Electrolyte Blood Press. 2013 Jun;11(1):29-32. https://synapse.koreamed.org/DOIx.php?id=10.5049/EBP.2013.11.1.29 http://www.ncbi.nlm.nih.gov/pubmed/23946763?tool=bestpractice.com [97]Park HS, Hong YA, Kim HG, et al. Usefulness of continuous renal replacement therapy for correcting hypernatremia in a patient with severe congestive heart failure. Hemodial Int. 2012 Oct;16(4):559-63. http://www.ncbi.nlm.nih.gov/pubmed/22515501?tool=bestpractice.com [98]Yang CW, Kim YS, Park IS, et al. Treatment of severe acute hypernatremia and renal failure by hemodialysis. Nephron. 1995;70(3):372-3. http://www.ncbi.nlm.nih.gov/pubmed/7477631?tool=bestpractice.com [99]Yang YF, Wu VC, Huang CC. Successful management of extreme hypernatraemia by haemofiltration in a patient with severe metabolic acidosis and renal failure. Nephrol Dial Transplant. 2005 Sep;20(9):2013-4. https://academic.oup.com/ndt/article/20/9/2013/1850324 http://www.ncbi.nlm.nih.gov/pubmed/15985507?tool=bestpractice.com Use of slow dialysis, in the form of continuous dialysis or sustained low-efficiency dialysis, has only been reported in patients with acute hypernatremia.
Hemodialysis and hemofiltration may correct the sodium level too quickly.[100]Ma F, Liu Y, Bai M, et al. The reduction rate of serum sodium and mortality in patients undergoing continuous venovenous hemofiltration for acute severe hypernatremia. Am J Med Sci. 2016 Jun 11;352(3):272-9. http://www.ncbi.nlm.nih.gov/pubmed/27650232?tool=bestpractice.com Adverse outcomes may be avoided by appropriate increases in the sodium level of the dialysate or replacement fluid, and reduction of the rate of correction by using slower blood and dialysate flows and lower-efficiency dialyzers (e.g., sustained low-efficiency dialysis).
Peritoneal dialysis using a low-sodium dialysate has been found to be effective in treating hypernatremia due to renal failure.[101]Moritz ML, del Rio M, Crooke GA, et al. Acute peritoneal dialysis as both cause and treatment of hypernatremia in an infant. Pediatr Nephrol. 2001 Sep;16(9):697-700. http://www.ncbi.nlm.nih.gov/pubmed/11511979?tool=bestpractice.com

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer