History and exam
Key diagnostic factors
common
presence of risk factors
Key risk factors include autoimmune disorders, lymphoproliferative disorders, mechanical prosthetic heart valves, family origin in Mediterranean, Middle East, sub-Saharan Africa, or Southeast Asia, family history of haemoglobinopathy or red blood cell membrane defects, paroxysmal nocturnal haemoglobinuria, thermal injury, or recent exposure to cephalosporins, penicillins, quinine derivatives, non-steroidal anti-inflammatory drugs, naphthalene, or fava beans.
pallor
Non-specific sign of anaemia.
jaundice
Due to increased bilirubin from red blood cell destruction.
Other diagnostic factors
common
fatigue
Non-specific sign of anaemia.
shortness of breath
Non-specific sign of anaemia.
dizziness
Non-specific sign of anaemia.
splenomegaly
Non-specific sign. May be present in hereditary spherocytosis, non-Hodgkin's lymphoma, or portal hypertension due to cirrhosis.[38]
uncommon
active infections
Direct parasitic destruction of the red blood cell (RBC) caused by agents such as malaria, Babesia, or Bartonella.[32][33]
Infection-related toxins can produce direct lysis of RBC, as in clostridial sepsis.
Also associations with pneumococci, Escherichia coli, and staphylococci.
May be present in mild or localised infection as well as in systemic, severe infection.
episodic dark urine (haemoglobinuria)
Suggestive of paroxysmal nocturnal haemoglobinuria.
triggered by exposure to cold
Donath-Landsteiner antibody (cold agglutinins) may be present.
Risk factors
strong
autoimmune disorders
Systemic lupus erythematosus (SLE), rheumatoid arthritis, and scleroderma are associated with an increased incidence of warm autoimmune haemolytic anaemia due to autoantibody production.[10][11] The incidence of clinically significant haemolytic anaemia has been described in up to 10% of patients with SLE.[13] On a lesser scale, other entities such as rheumatoid arthritis, scleroderma, dermatomyositis, and inflammatory bowel disease have an increased incidence of autoantibody formation.[14]
lymphoproliferative disorders
Oncology patients, specifically patients with chronic lymphocytic leukaemia (CLL), non-Hodgkin's lymphoma (NHL), and plasma cell disorders, often develop warm autoimmune haemolytic anaemia (AIHA).[15][16][17][18][19]
Estimates of the incidence of AIHA are 7% to 10% in patients with CLL and 3% in patients with NHL.[15][16]
prosthetic heart valve
family origin in Mediterranean, Middle East, Africa, or Southeast Asia
Haemoglobinopathies (thalassaemia, sickle cell anaemia) are more common in groups originating in these areas.[23][24]
Africa is the World Health Organization region with the highest incidence of glucose-6-phosphate dehydrogenase deficiency; it is very common in the sub-Saharan region.[3][4][Figure caption and citation for the preceding image starts]: Digitally-colourised scanning electron micrograph showing normal red blood cell (RBCs) and a sickle cell RBC (left) in a blood specimen of patient with sickle cell anaemiaCDC/ Sickle Cell Foundation of Georgia: Jackie George, Beverly Sinclair [Citation ends].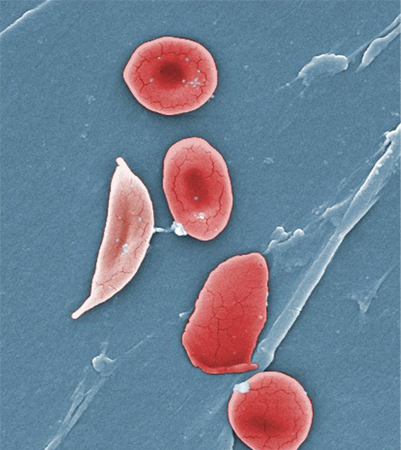
family history of haemoglobinopathy or red blood cell membrane defects
Suggests heritable causes of haemolysis, such as glucose-6-phosphate dehydrogenase deficiency, thalassaemia, or sickle cell anaemia.[1]
paroxysmal nocturnal haemoglobinuria
A rare disorder resulting in an acquired red blood cell membrane defect and subsequent haemolysis. Characterised by haemolysis, increased risk of thrombosis, and bone marrow failure.[25]
recent exposure to cephalosporins, penicillins, quinine derivatives, or non-steroidal anti-inflammatory drugs
Many drugs have been implicated in immune responses resulting in haemolysis.[12][26] Cephalosporins in particular have been associated with significant haemolysis, including fatal cases.[27] If haemolytic anaemia is diagnosed and drugs are suspected as the inciting event, a comprehensive search of the patient's medications should be conducted.
recent exposure to naphthalene or fava beans
May trigger haemolysis in glucose-6-phosphate dehydrogenase (G6PD) deficiency.[28] Naphthalene is found in moth balls.
G6PD deficiency is the most common deficiency of erythrocyte enzymes that results in a shortening of the erythrocyte lifespan.
thermal injury
Can cause haemolysis by disruption of red cells by microangiopathic processes.
weak
exceptional exertion
Circulatory trauma may lead to footstrike (march) haemolysis.[29]
recent exposure to nitrites, dapsone, ribavirin, or phenazopyridine
Medications can induce non-immune destruction of red blood cells, usually by oxidative stress.[30]
recent paraquat ingestion
Herbicides can induce non-immune destruction of red blood cells, usually by oxidative stress.[31]
malaria
Direct interaction between the infective organism and the red blood cell is responsible for haemolysis.[32][Figure caption and citation for the preceding image starts]: A photomicrograph of a blood smear showing erythrocytes containing developing Plasmodium vivax parasitesCDC/ Dr Mae Melvin [Citation ends].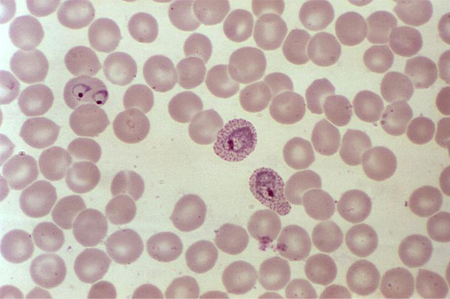
babesiosis
Direct interaction between the infective organism and the red blood cell is responsible for haemolysis.[33][Figure caption and citation for the preceding image starts]: Photomicrograph revealing the presence of what were determined to be numbers of intraerythrocytic Babesia sp. ring-form parasitesCDC/ Dr Mae Melvin [Citation ends].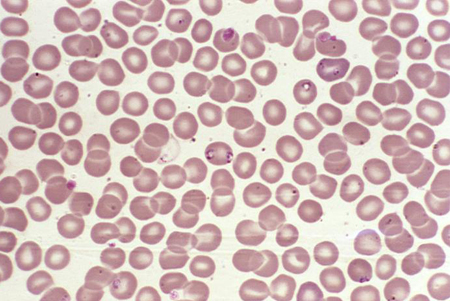
bartonellosis
Direct interaction between the infective organism and the red blood cell is responsible for haemolysis.
leishmaniasis
Direct interaction between the infective organism and the red blood cell is responsible for haemolysis.
Clostridium perfringens infection
Haemophilus influenzae type B infection
Capsular polysaccharide binds with the red blood cell membrane, and infected patients form antibodies to this complex.[36]
liver disease
May be associated with splenomegaly and haemolysis. Alcoholic cirrhosis in particular results in structural changes to the red blood cell membrane and subsequent haemolysis.
Use of this content is subject to our disclaimer