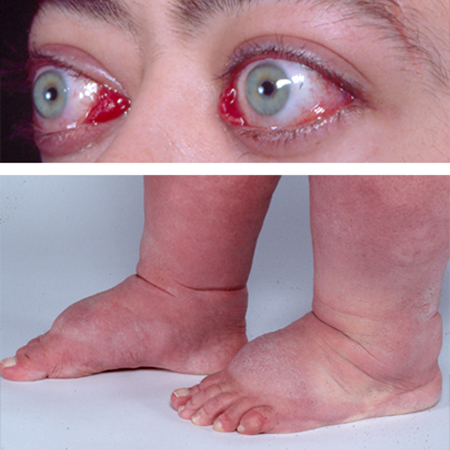
Graves’ orbitopathy occurs in around 25% of cases and is usually mild.[1]Tanda ML, Piantanida E, Liparulo L, et al. Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013 Apr;98(4):1443-9.
https://academic.oup.com/jcem/article/98/4/1443/2536784
http://www.ncbi.nlm.nih.gov/pubmed/23408569?tool=bestpractice.com
It is important to assess and classify the activity and severity of orbitopathy to help guide treatment.[58]Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67.
https://www.doi.org/10.1530/EJE-21-0479
http://www.ncbi.nlm.nih.gov/pubmed/34297684?tool=bestpractice.com
[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
Guidelines providing recommendations for initial assessment and management have been published.[58]Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67.
https://www.doi.org/10.1530/EJE-21-0479
http://www.ncbi.nlm.nih.gov/pubmed/34297684?tool=bestpractice.com
[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
[156]Perros P, Dayan CM, Dickinson AJ, et al. Management of patients with Graves' orbitopathy: initial assessment, management outside specialised centres and referral pathways. Clin Med (Lond). 2015 Apr;15(2):173-8.
https://www.rcpjournals.org/content/clinmedicine/15/2/173
http://www.ncbi.nlm.nih.gov/pubmed/25824071?tool=bestpractice.com
In children, orbitopathy is frequently mild and will often regress once euthyroid status is restored. A 'wait and see' strategy may be appropriate.
Risk factors should be addressed to prevent progression of Graves’ orbitopathy, including ensuring adequate control of thyroid dysfunction and supporting patients who smoke to stop.[58]Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67.
https://www.doi.org/10.1530/EJE-21-0479
http://www.ncbi.nlm.nih.gov/pubmed/34297684?tool=bestpractice.com
Mildly active orbitopathy is managed with lubricant eye drops and ointments, or sunglasses for surface symptoms, or prisms for diplopia.[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
Some patients with mild Graves’ orbitopathy of short duration may benefit from a 6-month course of selenium supplements.[157]Marcocci C, Kahaly GJ, Krassas GE, et al. Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011 May 19;364(20):1920-31.
http://www.nejm.org/doi/full/10.1056/NEJMoa1012985#t=article
http://www.ncbi.nlm.nih.gov/pubmed/21591944?tool=bestpractice.com
[158]Bednarczuk T, Schomburg L. Challenges and perspectives of selenium supplementation in Graves' disease and orbitopathy. Hormones (Athens). 2020 Mar;19(1):31-9.
https://www.doi.org/10.1007/s42000-019-00133-5
http://www.ncbi.nlm.nih.gov/pubmed/31721133?tool=bestpractice.com
Patients with active moderate-to-severe orbitopathy should be managed by an expert multidisciplinary team consisting of ophthalmologists and endocrinologists; first-line treatment is intravenous corticosteroid pulse-dose therapy (e.g., methylprednisolone).[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
[159]Zang S, Ponto KA, Kahaly GJ. Clinical review: intravenous glucocorticoids for Graves' orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011 Feb;96(2):320-32.
http://www.ncbi.nlm.nih.gov/pubmed/21239515?tool=bestpractice.com
[160]Bartalena L, Krassas GE, Wiersinga W, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves' orbitopathy. J Clin Endocrinol Metab. 2012 Dec;97(12):4454-63.
https://academic.oup.com/jcem/article/97/12/4454/2536457
http://www.ncbi.nlm.nih.gov/pubmed/23038682?tool=bestpractice.com
Use of other agents, including a combination of a corticosteroid plus mycophenolate, or targeted immunotherapy (e.g., teprotumumab) is recommended in some countries.[58]Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. 2021 Aug 27;185(4):G43-G67.
https://www.doi.org/10.1530/EJE-21-0479
http://www.ncbi.nlm.nih.gov/pubmed/34297684?tool=bestpractice.com
[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
[161]American Academy of Ophthalmology. Thyroid eye disease. Jul 2024 [internet publication].
https://eyewiki.org/Thyroid_Eye_Disease
[162]Men CJ, Kossler AL, Wester ST. Updates on the understanding and management of thyroid eye disease. Ther Adv Ophthalmol. 2021 Jan-Dec;13:25158414211027760.
https://www.doi.org/10.1177/25158414211027760
http://www.ncbi.nlm.nih.gov/pubmed/34263138?tool=bestpractice.com
[163]Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017 May 4;376(18):1748-1761.
https://www.doi.org/10.1056/NEJMoa1614949
http://www.ncbi.nlm.nih.gov/pubmed/28467880?tool=bestpractice.com
[164]Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020 Jan 23;382(4):341-352.
https://www.doi.org/10.1056/NEJMoa1910434
http://www.ncbi.nlm.nih.gov/pubmed/31971679?tool=bestpractice.com
[165]Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021 Jun;9(6):360-72.
https://www.doi.org/10.1016/S2213-8587(21)00056-5
http://www.ncbi.nlm.nih.gov/pubmed/33865501?tool=bestpractice.com
[166]Feng W, Hu Y, Zhang C, et al. Efficacy and safety of mycophenolate mofetil in the treatment of moderate to severe Graves' orbitopathy: a meta-analysis. Bioengineered. 2022 Jun;13(6):14719-29.
https://www.tandfonline.com/doi/full/10.1080/21655979.2022.2101191#d1e361
http://www.ncbi.nlm.nih.gov/pubmed/35959915?tool=bestpractice.com
Adverse effects associated with teprotumumab include inflammatory bowel disease, otological symptoms including hearing loss, and hyperglycaemia.[165]Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021 Jun;9(6):360-72.
https://www.doi.org/10.1016/S2213-8587(21)00056-5
http://www.ncbi.nlm.nih.gov/pubmed/33865501?tool=bestpractice.com
[167]Kossler AL, Douglas R, Dosiou C. Teprotumumab and the evolving therapeutic landscape in thyroid eye disease. J Clin Endocrinol Metab. 2022 Aug 8;107(suppl_1):S36-46.
https://academic.oup.com/jcem/article/107/Supplement_1/S36/6658346
http://www.ncbi.nlm.nih.gov/pubmed/36346685?tool=bestpractice.com
Monoclonal antibody therapies (e.g., rituximab and tocilizumab) have shown mixed results and are considered second- or third-line options.[155]Burch HB, Perros P, Bednarczuk T, et al. Management of thyroid eye disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022 Dec;32(12):1439-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9807259
http://www.ncbi.nlm.nih.gov/pubmed/36480280?tool=bestpractice.com
[168]Salvi M, Vannucchi G, Currò N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015 Feb;100(2):422-31.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318899
http://www.ncbi.nlm.nih.gov/pubmed/25494967?tool=bestpractice.com
[169]Stan MN, Garrity JA, Carranza Leon BG, et al. Randomized controlled trial of rituximab in patients with Graves' orbitopathy. J Clin Endocrinol Metab. 2015 Feb;100(2):432-41.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318907
http://www.ncbi.nlm.nih.gov/pubmed/25343233?tool=bestpractice.com
[170]Kang S, Hamed Azzam S, Minakaran N, et al. Rituximab for thyroid-associated ophthalmopathy. Cochrane Database Syst Rev. 2022 Jun 16;6(6):CD009226.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009226.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/35709102?tool=bestpractice.com
Orbital irradiation, with or without corticosteroids, is also used for moderate-to-severe active orbitopathy; there is limited evidence to suggest that orbital radiation may prevent compressive optic neuropathy.[171]Chundury RV, Weber AC, Perry JD. Orbital radiation therapy in thyroid eye disease. Ophthal Plast Reconstr Surg. 2016 Mar-Apr;32(2):83-9.
http://www.ncbi.nlm.nih.gov/pubmed/26325378?tool=bestpractice.com
[172]Rajendram R, Bunce C, Lee RW, et al. Orbital radiotherapy for adult thyroid eye disease. Cochrane Database Syst Rev. 2012 Jul 11;(7):CD007114.
http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007114.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/22786503?tool=bestpractice.com
[173]Tanda ML, Bartalena L. Efficacy and safety of orbital radiotherapy for Graves' orbitopathy. J Clin Endocrinol Metab. 2012 Nov;97(11):3857-65.
http://www.ncbi.nlm.nih.gov/pubmed/22962421?tool=bestpractice.com
Rehabilitative surgery has an important role in moderate-to-severe orbitopathy when the disease is stable and inactive.