FDA approves palopegteriparatide for hypoparathyroidism in adults
The US Food and Drug Administration (FDA) has approved palopegteriparatide for the treatment of hypoparathyroidism in adults. It is the first and only treatment approved for hypoparathyroidism.
Palopegteriparatide is a prodrug consisting of parathyroid hormone (1‐34) conjugated to a methoxypolyethylene glycol (mPEG) carrier.
In a 26-week phase 3, double-blind trial of adults with chronic hypoparathyroidism, 79% of patients randomised to palopegteriparatide, and 5% to placebo, maintained normal calcium levels without needing conventional therapy (i.e., active vitamin D and high doses of calcium).[36]
Palopegteriparatide is approved in Europe for the treatment of chronic hypoparathyroidism in adults.
Summary
Definition
History and exam
Key diagnostic factors
- history of thyroid, parathyroid, or laryngeal surgery
- chronic alcoholism
- malnutrition, malabsorption, diarrhoea
- muscle twitches, spasms, cramps
- paraesthesias, numbness, tingling
- poor memory, slowed thinking
- Chvostek's sign
- convulsions
- irregular heart beat, tachycardia
- Trousseau's sign
Other diagnostic factors
- anxiety
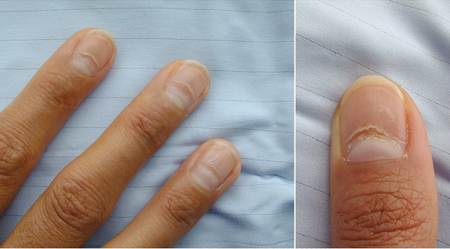
- dry hair, brittle nails
- cataracts
- history of mucocutaneous candidiasis
- history of chronic transfusions in patients with thalassaemia
- dyspnoea
- laryngeal spasm
Risk factors
- thyroid surgery
- parathyroid surgery
- hypomagnesaemia
- moderate and chronic maternal hypercalcaemia (neonatal hypocalcaemia)
- autosomal dominant conditions (e.g., mutations in CASR, GATA3)
- hereditary haemochromatosis
- transfusional iron overload in thalassaemia
- Wilson's disease
- metastatic cancer
Diagnostic investigations
1st investigations to order
- serum calcium
- plasma intact PTH
- serum albumin
- serum magnesium
- serum 25-hydroxyvitamin D
- serum phosphorus
- serum creatinine
- ECG
Investigations to consider
- 24-hour urine calcium, creatinine
- 24-hour magnesium, creatinine
- liver function tests
- arterial blood gases (ABGs)
- serum free thyroxine, thyrotropin
- morning cortisol and adrenocorticotrophin (ACTH) stimulation testing
- full blood count
- serum iron, transferrin, ferritin
- serum copper
- ophthalmological examination
- audiology
- renal imaging
- autoantibodies to type 1 interferon or 21-hydroxylase
- gene sequencing
Treatment algorithm
Contributors
Authors
Dolores Shoback, MD
Professor of Medicine
University of California San Francisco
Endocrine Research Unit
San Francisco VA Medical Center
San Francisco
CA
Disclosures
DS declares that her institution receives research money from Ascendis Pharmaceuticals to conduct a clinical trial in hypoparathyroid patients on which she is the Principal Investigator. This company is testing a new treatment for hypoparathyroidism. DS has participated in an advisory board for Ascendis (Dec 2022) to review data on this new treatment.
Quan-Yang Duh, MD, FACS
Professor of Surgery
Section of Endocrine Surgery
University of California San Francisco
San Francisco
CA
Disclosures
QYD declares that he has no competing interests.
Acknowledgements
Professors Dolores Shoback and Quan-Yang Duh would like to gratefully acknowledge Professor Ronald Merrell, a previous contributor to this topic.
Disclosures
RM declares that he has no competing interests.
Peer reviewers
Wail Malaty, MD
Adjunct Clinical Professor
Department of Family Medicine
University of North Carolina
Chapel Hill
NC
Disclosures
WM declares that he has no competing interests.
Use of this content is subject to our disclaimer