Approach
Treatment is guided primarily by tumor grade and stage determined at initial resection, along with the finding of additional laboratory and imaging investigations. Accurate staging, which requires resection into detrusor muscle, is key.
Nonmuscle-invasive tumors
Low risk
American Urological Association (AUA) bladder cancer guidelines define low-risk bladder cancer as: solitary, small-volume (≤3 cm), low-grade Ta disease (Ta = noninvasive papillary carcinoma); any papillary urothelial neoplasm of low malignant potential.[40]
Transurethral resection of a bladder tumor (TURBT) is first-line therapy. Guidelines recommend repeat transurethral resection within 6 weeks to lower recurrence if the initial resection was incomplete.[40][47]
An immediate, single instillation of intravesical chemotherapy (administered within 24 hours of transurethral resection) is recommended to reduce the risk of recurrence.[40] Instillation should not be done if bladder perforation is suspected or resection is extensive. Gemcitabine and mitomycin are commonly used.[43] Gemcitabine is preferred; it has favorable tolerability and may reduce the risk of recurrence and progression over time compared with mitomycin.[43][81] Epirubicin is an alternative option.[40][43][82][Figure caption and citation for the preceding image starts]: Low-grade, noninvasive (Ta) papillary urothelial carcinoma. Note adjacent satellite tumor, illustrating the field effectFrom the collection of Donald Lamm, MD, FACS [Citation ends].
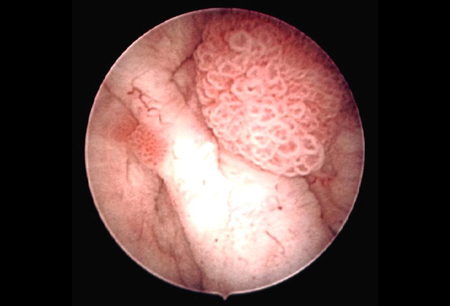
Intermediate risk
AUA intermediate-risk patients are those with large-volume (>3 cm) or multifocal low-grade Ta disease; high-grade Ta disease ≤3 cm; or low-grade T1 disease; or recurrence of Ta tumor within 1 year (T1 = tumor invades subepithelial connective tissue, i.e., the lamina propria).[40] These patients have a high risk of recurrence but a low risk of disease progression.
TURBT is first-line therapy. Guidelines recommend repeat transurethral resection within 6 weeks to lower recurrence if the initial resection was incomplete, there is no detrusor muscle in the initial resection specimen, or if T1 tumors are found.[40][47]
An immediate, single instillation of intravesical chemotherapy (administered within 24 hours of transurethral resection) is recommended to reduce the risk of recurrence.[40][82][83] Instillation should not be done if bladder perforation is suspected or resection is extensive. Gemcitabine and mitomycin are commonly used.[43] Gemcitabine is preferred; it has favorable tolerability and may reduce the risk of recurrence and progression over time compared with mitomycin.[43][81][83] Epirubicin is an alternative option.[40][43][82] BCG is never appropriate for immediate postoperative instillation owing to the risk of sepsis.[84]
Delayed intravesical BCG immunotherapy or intravesical chemotherapy may be considered for patients with intermediate-risk disease, starting 3-4 weeks after TURBT and administered every week for 6 weeks.[40][43] Decisions about additional intravesical therapy are based on assessment of risk of recurrence, patient history and symptoms, risks of adverse outcomes from repeat resection, and toxicity of therapy.[40]
Maintenance therapy is an option if there is a complete response to delayed treatment.[40][43] The optimal duration of maintenance therapy is not known. Guidelines specify using BCG maintenance for 1 year in intermediate-risk disease.[40][43][85] A 3-week BCG regimen given at 3, 6, and 12 months is commonly used.[43][86]
Patients with persistent or recurrent disease or positive cytology following intravesical BCG immunotherapy may be offered a second course of BCG.[40]
Mitomycin and gemcitabine are alternatives for delayed intravesical chemotherapy. Other options include sequential gemcitabine plus docetaxel, epirubicin, valrubicin, docetaxel, or sequential gemcitabine plus mitomycin.[43] Docetaxel is well tolerated intravesically and is an effective option for BCG-refractory nonmuscle-invasive bladder cancer alone and in combination with gemcitabine.[87][88] Chemotherapy maintenance is commonly given at monthly intervals for 6-12 months.[40]
High risk
Defined as: carcinoma in situ; high-grade Ta >3 cm or multifocal; high-grade T1; any recurrent high-grade Ta tumor; any BCG failure in a high-grade patient; any variant histology or lymphovascular or prostatic urethral invasion.[40]
Transurethral resection, followed by induction and maintenance BCG immunotherapy, is first-line therapy.[40]
While not confirmed to be beneficial in high-risk disease, immediate, single, postoperative (within 24 hours) instillation of intravesical chemotherapy (e.g., gemcitabine, mitomycin, epirubicin) is sometimes used in addition to delayed intravesical immunotherapy.[40][89][90] BCG is never appropriate for immediate postoperative instillation owing to the risk of sepsis.[84]
Completeness of tumor resection, recurrence at 3 months, and the presence of residual disease at repeat resection all have important prognostic significance.[91][92]
Guidelines recommend repeat transurethral resection within 6 weeks to lower recurrence if the initial resection was incomplete, there is no detrusor muscle in the initial resection specimen, T1 tumors are found, or tumors have variant histology (and the patient is not having cystectomy).[40][47]
Repeat resection should also be considered for high-risk, high-grade Ta tumors.[40][93][94] Repeat resection will disclose residual tumor in about 50% to 70% of patients with T1 tumors.[40] Repeat resection demonstrating residual T1 disease has been associated with increased incidence of muscle invasion compared with no or non-T1 tumor detected on restaging transurethral resection.[95]
Intravesical BCG immunotherapy is most commonly given 3-4 weeks after transurethral resection and retained for 2 hours. Induction is weekly BCG for 6 weeks.[40][43] Maintenance BCG is recommended if there is a complete response to induction. The optimal duration of maintenance therapy is not known. Guidelines specify using BCG maintenance for 3 years, if tolerated, for high-risk disease.[40][85] Maintenance therapy is usually given in weekly instillations for 3 weeks at 3, 6, 12, 18, 24, 30, and 36 months. Dose reductions may be used if there are local symptoms or to prevent escalation of BCG adverse effects.[86] In high-risk patients, full-dose 3-year BCG reduces recurrences compared with full-dose BCG for 1 year.[85] Patients with persistent or recurrent disease after a single course of induction intravesical BCG should be offered a second course of BCG.[40]
Cystectomy constitutes over-treatment in most high-risk patients who do not have muscle invasion. National Comprehensive Cancer Network guidelines suggest that cystectomy is preferred for patients with very-high-risk features, defined as BCG unresponsiveness, variant histologies (e.g., micropapillary, plasmacytoid, sarcomatoid), lymphovascular invasion, and prostatic urethral invasion.[43] BCG is the preferred treatment option for high-risk patients (i.e., without very-high-risk features).[43] Care must be taken when selecting patients for cystectomy, especially in the absence of muscle invasion; overall 90-day mortality is as high as 9%, and increased age, American Society of Anesthesiology score, and presence of lymph-node or distant metastasis increase mortality.[96]
High risk: BCG-unresponsive or intolerant
Cystectomy is the preferred treatment for patients who are BCG intolerant or have BCG-unresponsive disease (recurrence or progression during or following adequate BCG therapy).[43] Other options may include intravesical chemotherapy, pembrolizumab, or nadofaragene firadenovec. National Comprehensive Cancer Network (NCCN) guidelines include nogapendekin alfa inbakicept (an immune cell-activating interleukin-15 superagonist) plus BCG as an additional option for select patients.[43]
Optimal management for patients who decline or are unfit for cystectomy has not been established. Patients should be offered enrollment in a clinical trial or an alternative intravesical therapy, such as mitomycin, gemcitabine, or gemcitabine plus docetaxel.[40][47]
Pembrolizumab, nadofaragene firadenovec, and nogapendekin alfa inbakicept plus BCG are approved by the Food and Drug Administration (FDA) for patients with BCG-unresponsive high-risk nonmuscle-invasive carcinoma in situ.[43][97][98][99] The pembrolizumab approval stipulates that patients are ineligible for or elect not to undergo cystectomy. Systemic immunotherapy with pembrolizumab is given to a patient with carcinoma in situ within 12 months of completion of adequate BCG therapy.[40]
Patients with coexisting obstructive benign prostatic hyperplasia (BPH)
Patients with nonmuscle-invasive bladder tumors and coexisting obstructive BPH may have transurethral resection of the prostate at the same time as TURBT. Meta-analysis demonstrates that performing the procedures simultaneously improves patient quality of life, without any risk of increasing tumor recurrence or metastasis rates.[100] This approach is appropriate for low, intermediate, and high-risk nonmuscle-invasive bladder tumors.
Locally invasive tumors
Localized muscle-invasive disease: T2a-T4a N0M0 or N1
Guidelines recommend neoadjuvant chemotherapy followed by cystectomy for eligible patients with T2-T4a disease without lymph node involvement (N0) or with involvement in a single pelvic lymph node (N1).[43][53][101] Neoadjuvant platinum-based combination chemotherapy reduces mortality risk without increased perioperative complications or mortality.[102][103] Dose-dense methotrexate plus vinblastine plus doxorubicin plus cisplatin (ddMVAC) is the preferred regimen; toxicity and efficacy are improved compared with traditional MVAC. Gemcitabine plus cisplatin may be an alternative option.[43][104][105][106][107][108]
Radical cystoprostatectomy (in men) or radical cystectomy often accompanied by hysterectomy (in women) is generally required, and is thought to provide the best chance of cure. Bilateral pelvic lymph node dissection (PLND) is an essential part of the procedure. Extended node dissection is controversial; evidence of improved survival is equivocal while risk of adverse effects is increased.[43][53][109][110] Severe scarring secondary to previous surgery or treatments, advanced age, or severe comorbidities may preclude PLND. Cystectomy is followed by the formation of a urinary diversion by means of an ileal conduit to the skin or by creating an internal reservoir that can be drained by catheter or through the urethra. Relative contraindications to urethral drainage include Tis (carcinoma in situ) in the prostatic ducts or a positive urethral margin.[43] An orthotopic neobladder provides some function similar to a native bladder but has an increased risk of night-time incontinence and retention requiring intermittent self-catheterization.
In selected patients with T2 disease, a partial cystectomy may be feasible.[43] This requires a solitary tumor, located in an area of the bladder where a minimum clear margin of 2 cm of noninvolved urothelium can be achieved as well as sufficient soft tissue to enable the tumor to be removed without significantly reducing bladder capacity or causing incontinence. Mostly this procedure is reserved for tumors in the dome of the bladder that have no associated Tis (carcinoma in situ) in other areas of the bladder. Relative contraindications are lesions in the trigone or bladder neck.
For patients who decline or are not candidates for cystectomy, trimodal organ-preservation therapy (TMT) with the combination of maximal TURBT, chemotherapy, and radiation therapy may be an alternative option.[53][111][112] Preferred candidates for organ preservation therapy include those with smaller solitary tumors, no nodal involvement, no extensive or multifocal carcinoma in situ, no hydronephrosis, and good pretreatment bladder function.[53] Adequate patient counseling of the risks and regular posttreatment surveillance are essential. While one meta-analysis found that TMT was noninferior to radical cystectomy at <10 years; overall, TMT was associated with an increased risk of all-cause and bladder-specific cancer mortality.[113] About 30% of patients treated with multimodal organ-preservation therapy will have recurrent invasive disease and require subsequent radical cystectomy.[111]
Postcystectomy chemotherapy or chemoradiation therapy may be considered in select high-risk patients (e.g., pathologic T3-4, positive nodes, positive margins).[43][114][115] A cisplatin-based combination such as ddMVAC, or gemcitabine plus cisplatin may be used.[43] Data regarding the use of postcystectomy radiation therapy and chemoradiation therapy are limited.[43][53][111] One systematic review reported no clear benefit attributable to adjuvant radiation therapy following cystectomy.[116] In a randomized phase 2 trial of postcystectomy patients with locally advanced bladder cancer (urothelial carcinoma or squamous cell carcinoma), adjuvant sequential chemotherapy and radiation therapy significantly improved local control compared with adjuvant chemotherapy alone (96% vs. 69%, respectively, at 2 years).[117] Disease-free and overall survival did not differ significantly between treatment groups.
Postcystectomy nivolumab may be considered for select high-risk patients with residual disease who are not eligible for, or who decline, cisplatin-based adjuvant therapy.[43][53] In one randomized controlled trial, postoperative nivolumab significantly improved disease-free survival at 6 months compared with placebo (HR 0.70); the increase in survival was greater among patients with a PD-L1 expression ≥1% (HR 0.55). Patients in the trial had pathological evidence of urothelial carcinoma with a high risk of recurrence.[118]
Unresectable locally advanced: T4b or N2-3
T4b and N2-3 disease is typically considered unresectable (defined by fixed bladder mass or positive nodes evident before laparotomy) and is generally treated by chemotherapy alone, or chemoradiation therapy.[43] For patients who show no nodal disease on CT scans, 2 or 3 courses of chemotherapy with or without radiation therapy is recommended, followed by cystoscopy and repeat CT scan.[43] If the tumor responds to treatment, subsequent options include cystectomy or consolidation chemotherapy with or without radiation therapy. Contraindications to radiation include previous pelvic radiation, contracted bladder, or irritative bladder symptoms.
Maintenance avelumab is recommended following completion of chemotherapy for patients with good response and no disease progression.[43] In a phase 3 trial, maintenance avelumab increased overall survival by 7.1 months compared with supportive therapy.[119]
A number of immunotherapies are approved for patients with locally advanced or metastatic disease as
alternative first-line treatment, or
second-line treatment for patients with progression during or following platinum-based chemotherapy.
These options are not commonly used for nonmetastatic disease in practice. See the section on metastatic disease below for further discussion of immunotherapy.
Metastatic disease
Patients who present with metastatic disease, or subsequently develop metastatic disease, are generally treated with systemic therapy.[43][53] Palliative radiation therapy, usually in combination with systemic therapy, can be used to reduce symptoms or improve local control. The therapy regimen used may vary according to factors such as the presence and severity of comorbidities (e.g., cardiac disease, neuropathy, hearing loss, renal dysfunction), together with an assessment of risk based on extent of disease.
Molecular/genomic analysis, including testing for FGFR3 genetic alterations and HER2 overexpression, may help guide subsequent treatment options and/or eligibility for clinical trials.[43]
First-line systemic therapy
Guidelines recommend pembrolizumab plus the antibody-drug conjugate enfortumab vedotin as the preferred first-line treatment for patients with metastatic disease who are fit enough for combination therapy.[43][53][120] Improved survival outcomes have been reported with pembrolizumab plus enfortumab vedotin compared with cisplatin-based chemotherapy.[121][122]
For patients with metastatic disease who are not able to receive pembrolizumab plus enfortumab vedotin (e.g., due to contraindications or availability), recommended regimens for cisplatin-eligible patients include ddMVAC, or gemcitabine plus cisplatin, or gemcitabine plus cisplatin plus nivolumab.[43][53][120][123] Gemcitabine plus carboplatin is the preferred chemotherapy regimen for cisplatin-ineligible patients (i.e., those with any of: creatinine clearance <60 mL/min; Eastern Cooperative Oncology Group performance score 2; grade ≥2 neuropathy or hearing loss; New York Heart Association class III heart failure).[43][53][120][124][125] After 2 or 3 cycles of chemotherapy, patients are reevaluated and treatment is continued for up to 6 cycles in total if the disease has responded or remained stable.[43]
Further treatment after systemic therapy
Surgery or radiation therapy may be considered in highly selected patients who show a major partial response in an unresectable primary tumor or have a solitary site of residual disease that is resectable after chemotherapy.[43][53][126] If disease is completely resected, two additional cycles of chemotherapy can be given if tolerated by the patient.
Maintenance avelumab is recommended following completion of chemotherapy (without nivolumab) for patients with good response and no disease progression.[43][53][119] For patients receiving gemcitabine and cisplatin plus nivolumab, maintenance nivolumab is recommended.[43][53][123]
Enrollment into a clinical trial, if eligible, is strongly recommended for second-line and subsequent-line therapies for advanced and metastatic disease; evidence for optimal treatment selection is lacking. Choice of treatment should be based on prior therapy and cisplatin eligibility.
For patients who progress following first-line treatment with pembrolizumab plus enfortumab vedotin, guidelines recommend platinum-based chemotherapy or enfortumab vedotin monotherapy (if ineligible for cisplatin-based chemotherapy).[43][53][120]
Pembrolizumab (preferred), nivolumab, avelumab, or enfortumab vedotin may be used as second-line treatments in patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-based chemotherapy.[43][127][128]
The fibroblast growth factor receptor (FGFR) inhibitor erdafitinib is an alternative option for certain patients with susceptible FGFR3 genetic alterations who have received at least one line of prior systemic therapy.[43][53][120] Erdafitinib is approved in the US and Europe for locally advanced or metastatic urothelial carcinomas that express FGFR3 gene alterations and have progressed during or after at least one line of prior systemic therapy (which should include immunotherapy for eligible patients). In patients with progressive disease and at least one prespecified FGFR alteration, erdafitinib produced a 40% objective response rate in one phase 2 trial and an improvement in overall survival compared with chemotherapy in one phase 3 trial.[129][130]
Subsequent lines of therapy depend on prior therapy, and may include enfortumab vedotin, erdafitinib (if positive for FGFR3 genetic alterations), or chemotherapy. The FDA has approved fam-trastuzumab deruxtecan (a HER2-directed antibody-conjugate) for the treatment of HER2-positive unresectable or metastatic bladder tumors in patients who have received prior treatment (or who have no further alternative treatment options). In a phase 2, open-label trial, fam-trastuzumab deruxtecan treatment resulted in an objective response rate of 39% (16/41) and a median progression-free survival of 7 months in patients with bladder cancer with HER2 overexpression (immunohistochemistry 3+/2+).[131]
Use of this content is subject to our disclaimer