Etiology
Smoking is the most important causative factor in bladder cancer, with an attributable risk of around 50%.[11] The relative risk of bladder cancer in people who have any history of smoking versus those who have never smoked is around 2 to 3.[23] The relative risk of bladder cancer from second-hand smoke is 1.4.[23]
Occupational exposure to chemical carcinogens amounts to 5% to 6% of the attributable risk of bladder cancers and may include aromatic amines used in rubber and dye industries, and polycyclic aromatic hydrocarbons used in the aluminum, coal/oil/petroleum, and roofing industries.[11] Other occupational groups at increased risk include firefighters, painters, and hairdressers.[24] Pelvic radiation, as commonly used for prostate cancer, and chemotherapy such as cyclophosphamide significantly increase the risk of bladder cancer.[11][24] Environmental exposure to arsenic in drinking water is a recognized causative factor of bladder cancer.[11][25]
Evidence is conflicting, but people with diabetes (primarily type 2) may have an increased risk of bladder cancer.[11][26] Some evidence suggests that pioglitazone is associated with an increased risk of bladder cancer in adults with type 2 diabetes.[27]
Keratinizing squamous metaplasia, which appears as leukoplakia on cystoscopy, often develops as a result of chronic irritation/inflammation (e.g., chronic urinary tract infection, Schistosoma infection, chronic indwelling catheters) and increases the risk of squamous cell carcinoma of the bladder.[11][28]
Familial cases of bladder cancer also occur; 4.3% of bladder cancer patients have a first-degree relative with bladder cancer, and up to 50% of urothelial cancer patients have a family history of cancer.[11] Among patients suspected to have a familial cancer syndrome, germline pathogenic and likely pathogenic variants are most commonly found in BRCA1, MSH2, MLH1 , ATM, and CHEK2 genes.[29] Bladder cancer genetic susceptibility in people exposed to carcinogens is established.[11] Genes with increased frequency of alteration in muscle-invasive samples include TP53, PIK3CA, CDKN1A, ERCC2, fibroblast growth factor receptor pathway genes, and ERBB3.[30]
Pathophysiology
Carcinogens such as nitrosamines are concentrated and excreted in the urine, thereby exposing them to the cells lining the urinary tract. This exposure is prolonged in the bladder (where 95% of urothelial carcinomas arise) but malignant transformation can arise anywhere in the urinary tract, from the renal calyx to the urethral meatus.[31] It is likely that frequent and prolonged exposure to these carcinogens contributes to malignant transformation of urothelial cells. Studies of normal bladder tissue show extremely high rates of mutations with significant heterogeneity even in one bladder.[32]
Multifocal and synchronous tumors occur in 70% of newly diagnosed cases and in >50% of cases may be accompanied by areas of dysplasia up to carcinoma in situ, either in contiguous or remote areas. Metachronous recurrences occur in >60% of patients, often at sites remote from the primary. These findings led to the concept of a "field effect" in which exposure of the urothelium to carcinogens at roughly the same concentration gives rise to an epithelium from which occasional cells become initiated and give rise to independent clones of transformed cells. Subsequent promotion may lead to multifocal tumors, which may be synchronous or metachronous.
Although the field effect is thought to explain the multifocal nature of bladder cancer, X-chromosome inactivation studies suggest a single cell origin for most tumors.[33] However, both concepts have clinical utility: a point mutation may initiate the process, conferring a growth advantage to cells that later develop the malignant phenotype, perhaps explaining the proximity of new tumors, and the phenomena of cancer cells implanting downstream from upper tract tumors and implanting post-resection.
Genes participating in chemical detoxification such as glutathione S-transferase M1 and N-acetyltransferase play a role in development of bladder cancer. The first demonstration of an oncogene (ras) in human cells was in a bladder cancer cell line, but ras and other dominant oncogenes are infrequently involved. Loss of heterozygosity in the long arm of chromosome 9 (9q) is common in noninvasive tumors that have a favorable prognosis, while loss of tumor suppressor genes RB1 and TP53 is associated with invasive tumors and an unfavorable prognosis.[31] In muscle-invasive urothelial carcinoma, luminal and basal gene expression is not unlike that seen in breast cancer.[34]
Classification
TNM classification[3][4]
Based on biopsy results, physical exam, and imaging studies, bladder tumors are staged according to their depth of invasion. The most widely used and universally accepted staging system is the tumor-node-metastases (TNM) classification:
Size and extent of the primary tumor (T)
Regional lymph node involvement (N)
Presence or absence of distant metastases (M).
Under this system, nonmuscle-invasive bladder cancer includes:
Papillary tumors confined to the epithelial mucosa (Ta)
Tumors invading the subepithelial tissue (i.e., lamina propria; T1)
Carcinoma in situ (Tis).
Muscle-invasive bladder cancer includes:
Organ-confined tumors: invade muscularis propria (T2a or T2b)
Nonorgan-contained tumors: invade perivesical fat (T3a or T3b)
Tumors invade adjacent organs: prostate stroma, seminal vesicles, uterus, vagina, pelvic wall, abdominal wall (T4a or T4b).
World Health Organization (WHO) classification - urothelial carcinoma[5]
Noninvasive urothelial neoplasms
Urothelial papilloma
Inverted urothelial papilloma
Papillary urothelial neoplasm of low malignant potential (PUNLMP)
Noninvasive papillary urothelial carcinoma, low-grade
Noninvasive papillary urothelial carcinoma, high-grade
Urothelial carcinoma in situ
Invasive urothelial neoplasms
Invasive urothelial carcinoma
Tumors are graded according to their cellular characteristics. Previously, bladder carcinomas were graded according to the 1973 WHO grading of urothelial papilloma as well differentiated (G1), moderately differentiated (G2), or poorly differentiated (G3).[6] In 2004, the WHO and International Society of Urological Pathology (ISUP) published a grading system that used specific cytologic and architectural criteria.[7] The WHO/ISUP classification differentiates between papillary urothelial neoplasms of low-malignant potential and low-grade and high-grade urothelial carcinomas.
Further ISUP updates have clarified grading of noninvasive urothelial carcinoma with mixed grades and invasive urothelial carcinoma subtypes (variants) and divergent differentiations.[8] Although the WHO grading system has been updated, the older grading systems continue to be in use.[8][9]
Comparison of the 1973 and 2004 classification systems for noninvasive urothelial neoplasms:[10]
WHO 1973
Urothelial papilloma
Grade 1: well differentiated, have an existing papillary architecture, fine chromatin, and a little indication of nucleoli or mitoses
Grade 2: moderately differentiated, usually have a papillary architecture, granular chromatin, and a stronger indication of nucleoli and mitoses
Grade 3: poorly differentiated, least likely to have a papillary architecture, have coarse chromatin, and have many examples of nucleoli and mitoses.
WHO/ISUP 2004
Urothelial papilloma
PUNLMP
Low-grade papillary urothelial carcinoma
High-grade papillary urothelial carcinoma
Accurate staging and grading of bladder cancer is essential for proper diagnosis, treatment, and follow-up. Stage and grade of the cancer provides an indication of the likelihood of recurrence and the risk of progression to invasive cancer.[Figure caption and citation for the preceding image starts]: Low-grade, noninvasive (Ta) papillary urothelial carcinoma. Note adjacent satellite tumor, illustrating the field effectFrom the collection of Donald Lamm, MD, FACS [Citation ends].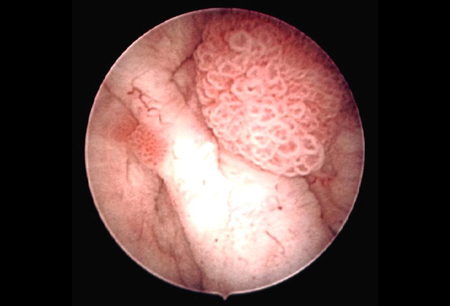 [Figure caption and citation for the preceding image starts]: Low-grade urothelial carcinoma seeding in the prostatic urethra; illustrated is the loop electrode used to resect bladder tumorsFrom the collection of Donald Lamm, MD, FACS [Citation ends].
[Figure caption and citation for the preceding image starts]: Low-grade urothelial carcinoma seeding in the prostatic urethra; illustrated is the loop electrode used to resect bladder tumorsFrom the collection of Donald Lamm, MD, FACS [Citation ends].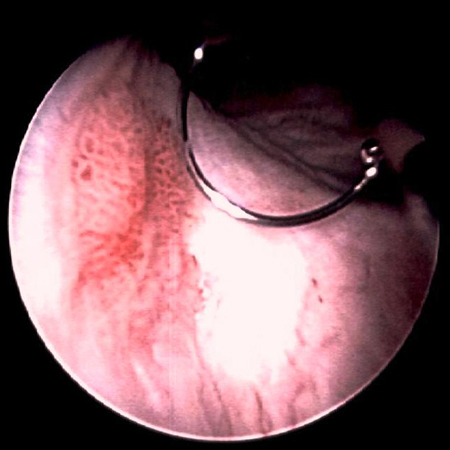 [Figure caption and citation for the preceding image starts]: Low-grade tumors surrounded by small satellite tumors with small, uniform fronds. In the foreground is a solid tumor on a broad base, a typical appearance of high-grade tumors. Low- and high-grade tumors often occur in the same patientFrom the collection of Donald Lamm, MD, FACS [Citation ends].
[Figure caption and citation for the preceding image starts]: Low-grade tumors surrounded by small satellite tumors with small, uniform fronds. In the foreground is a solid tumor on a broad base, a typical appearance of high-grade tumors. Low- and high-grade tumors often occur in the same patientFrom the collection of Donald Lamm, MD, FACS [Citation ends].
Use of this content is subject to our disclaimer