Tests
1st tests to order
complete blood count
Test
Baseline assessment as part of initial diagnostic workup.[15]
Result
may be anemia, thrombocytosis, thrombocytopenia, leukopenia
comprehensive metabolic panel
Test
To assess kidney, liver, electrolyte, glucose function; to evaluate for underlying organ dysfunction that may affect treatment.[15]
Result
may be abnormal
CT chest/abdomen/pelvis
Test
CT with intravenous contrast (unless contraindicated) for initial staging.[3][15]
CT abdomen and pelvis assesses for intra-abdominal disease, as well as pelvic lesions in women. [Figure caption and citation for the preceding image starts]: CT abdomen with intravenous contrast, revealing numerous enhancing lesions in the right hepatic lobe, with associated ascites. Percutaneous biopsy of one of these hepatic lesions revealed adenocarcinoma, but no primary site was identified during routine workup. This is a typical presentation of AUPFrom the personal collection of Dr D. Cosgrove [Citation ends].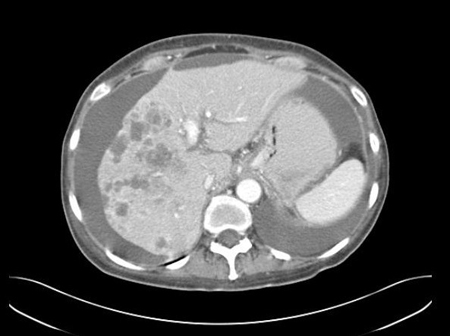 [Figure caption and citation for the preceding image starts]: CT abdomen with intravenous contrast, revealing numerous enhancing liver lesions in both hepatic lobes. Percutaneous biopsy of a right lobe lesion revealed adenocarcinomaFrom the personal collection of Dr D. Cosgrove [Citation ends].
[Figure caption and citation for the preceding image starts]: CT abdomen with intravenous contrast, revealing numerous enhancing liver lesions in both hepatic lobes. Percutaneous biopsy of a right lobe lesion revealed adenocarcinomaFrom the personal collection of Dr D. Cosgrove [Citation ends].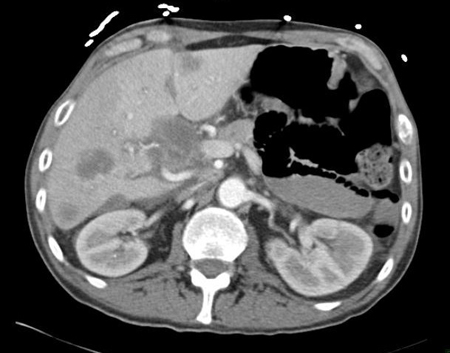
Result
abnormal masses
mammography
Test
Mammography is required for patients with clinical features compatible with breast cancer.[15]
The index of suspicion for breast carcinoma should be high in women with isolated axillary lymphadenopathy.
Breast MRI is indicated (without or with contrast [unless contraindicated]) if mammography is not diagnostic and/or in women with adenocarcinoma identified in axillary lymph nodes.[3][15]
Result
breast carcinoma is suggested by the presence of a mass lesion, or microcalcifications, on mammography
MRI of breast
Test
Breast MRI is indicated (without or with contrast [unless contraindicated]) if mammography is not diagnostic and/or in women with adenocarcinoma identified in axillary lymph nodes.[3][15]
Breast MRI may detect occult cancer in 50% of women with axillary metastases, irrespective of breast density.[38]
Result
breast carcinoma is suggested by the presence of a mass lesion on MRI
transvaginal ultrasound
Test
Transvaginal ultrasound is the preferred imaging modality for women presenting with ascites to assess for the presence of an ovarian mass. Color or power Doppler should be included in the examination.[31]
Result
ovarian carcinoma is suggested by the presence of an adnexal mass
diagnostic paracentesis
Test
Recommended (if technically possible) in all patients presenting with ascites.[24]
Women with ascites should undergo paracentesis to assess for peritoneal adenocarcinoma. Peritoneal adenocarcinoma with papillary features is a favorable clinicopathologic subtype.[2][27]
The favorable pathologic subtype of adenocarcinoma with papillary features is very rare in men.[28][29]
Larger volume (>1 liter) paracentesis increases the diagnostic yield.
Result
presence of adenocarcinoma cells in the peritoneal fluid
direct laryngoscopy with or without esophagoscopy and bronchoscopy
Test
Direct laryngoscopy with or without esophagoscopy and bronchoscopy is recommended for patients with cervical lymphadenopathy. The extent of examinations is dependent on the level of cervical nodes involved.
Additional immunohistochemical testing can help to confirm an occult head and neck primary tumor.
Identified lesions should be biopsied to confirm pathologic concordance with the metastatic focus. Treatment is definitive and potentially curative for patients with a localized head and neck primary tumor with locoregional cervical nodal metastases.[16]
Result
visualization of a mucosal lesion for primary head and neck carcinoma, esophageal carcinoma, or lung carcinoma
Tests to consider
fecal occult blood test
Test
Ordered if colorectal cancer is suspected.[15]
Result
may be positive
lactate dehydrogenase
urinalysis
Test
Can be used to assess renal function, detect paraneoplastic syndromes, or evaluate for associated metabolic abnormalities.[15]
Result
normal or abnormal
light microscopy, with hematoxylin and eosin (H&E) staining
Test
Light microscopy and standard hematoxylin and eosin staining of tissue from the (most accessible) metastatic focus can distinguish those tumors with obvious gland formation (well-differentiated to moderately differentiated adenocarcinoma) from those with scant glandular structures, consistent with adenocarcinoma due to mucin production (poorly differentiated or undifferentiated adenocarcinoma).
The degree of differentiation of the glandular structures is determined by the pathologist, and is typically classified as well-, moderately, poorly, or undifferentiated.[40]
Result
gland formation and presence of mucin
18F-fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT
Test
May be considered in patients presenting with a neck mass, as clinically indicated.[16]
FDG-PET/CT is not routinely recommended in evaluation of cancers of unknown primary; it may be considered in those for whom contrast enhancement is contraindicated.[3][15]
Result
may identify primary tumor site or other metastatic foci
immunohistochemical (IHC) markers
Test
IHC markers are employed to further categorize adenocarcinoma cells, in an attempt to identify a likely site of origin of the tumor.
A battery of markers is generally applied, although information from the history, physical exam, and initial testing will usually allow a more directed search.[15] Specific tests should be ordered based on indicators from the initial diagnostic workup and in consultation with a pathologist.
Pan-keratin (AE1/AE3 and CAM5.2): can indicate a carcinoma lineage.
Cytokeratin-7 (CK7): expression is generally limited to lung, breast, ovary, endometrium, and upper gastrointestinal/pancreaticobiliary cancers, and is not seen in lower gastrointestinal tract tumors.
Cytokeratin-20 (CK20): generally expressed on gastrointestinal epithelium, urothelium, and Merkel cells.
CDX2 or SATB2: suggest colorectal or other gastrointestinal primary.
TTF-1: suggestive of lung or thyroid primary.
Napsin A: suggests lung primary.
GATA3: suggests breast/urinary bladder/salivary gland primary.
A more extensive list of IHC markers is available in published guidelines.[15][19]
Result
specific tumor antigens are identified via labeled antibodies
estrogen and progesterone receptor status
Test
Immunohistochemical markers for estrogen receptor (ER) and progesterone receptor (PR) should be performed in all women with adenocarcinoma, especially in those with isolated axillary lymphadenopathy.
Tumors exhibiting these receptors are sensitive to hormonal therapy.
ER and PR expression can occasionally be seen in carcinomas of nonmammary/nongynecologic origin, and for this reason the diagnosis of metastatic breast, ovarian, or endometrial carcinoma in carcinoma of unknown primary site should not be based solely on expression.[41]
Result
Specific tumor receptors are identified via labeled antibodies
serum tumor markers
Test
Assessment of serum tumor markers may be considered in specific clinical scenarios. However, they are nonspecific and have no diagnostic role in patients with adenocarcinoma of unknown primary (AUP).
Prostate-specific antigen (PSA): men over the age of 40 with AUP, and all men with AUP and bone metastases, should have PSA checked to assess for potential prostate carcinoma.
Serum alpha-fetoprotein (AFP)/beta-human chorionic gonadotropin (beta-hCG): may be indicative of germ cell tumors, and should be checked in patients with mediastinal tumors and in men under the age of 65 presenting with a retroperitoneal mass.
Patients with germ cell tumors can be definitively treated with appropriate chemotherapy for curative intent, even if disseminated. AFP can also be useful if hepatocellular carcinoma is a potential diagnosis.
CA 125 is typically elevated in women with histology consistent with ovarian cancer.
Result
may show elevated PSA, AFP, beta-hCG, CA 125
next-generation sequencing (NGS)
Test
Can identify potentially actionable mutations and aberrations (e.g., neurotrophic tyrosine receptor kinase [NTRK] gene fusions), and should be considered after initial determination of histology is completed.[15]
Testing for predictive biomarkers (including tumor mutation burden [TMB] and assessment of microsatellite instability resulting from deficient DNA mismatch repair [MSI/dMMR]) for immune checkpoint inhibitors is recommended.
Initial results in patients with unfavorable cancer of unknown primary are promising.[32]
Result
presence of clinical biomarker(s) associated with response to targeted therapy and immunotherapy (e.g., NTRK gene fusions, TMB, MSI/dMMR)
Emerging tests
gene expression profiling (GEP)
Test
Encompasses molecular assays that employ messenger RNA-, DNA-, or microRNA-based platforms to identify cancers of unknown primary.
The technology is premised on the assumption that the molecular profile of the metastatic tumor(s) has a similar profile to that of the primary tumor. Surrogate measures (e.g., immunohistochemistry results) are typically used to determine assay accuracy because the primary site of adenocarcinoma of unknown primary site (AUP) is typically difficult to determine.
Observational studies suggest clinical utility.[33][34] However, recent prospective randomized clinical trials and meta-analyses of (prospective and retrospective) studies investigating the role of molecularly defined site-specific treatment of cancers of unknown primary have failed to report significant improvement in overall survival compared with empiric therapy.[35][36]
The clinical benefits of GEP testing remain to be determined; guidelines do not currently recommend routine GEP testing in patients with AUP.[2][15]
Result
determines tissue of origin; directs site-specific therapy
Use of this content is subject to our disclaimer