Approach
A universal definition of heart failure (HF), proposed in 2021 by the Heart Failure Society of America, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, describes HF as "a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion."[1] Identification of the condition responsible for the cardiac structural and/or functional abnormalities may be important, because some conditions that lead to left ventricular dysfunction are potentially treatable and/or reversible.[7]
Precipitating factors and comorbidity
Efforts to identify a cause frequently allow the detection of coexistent conditions that may contribute to or exacerbate the severity of symptoms. However, it may not be possible to discern the cause of HF in many patients presenting with this syndrome, and in others, the underlying condition may not be amenable to treatment.
A number of precipitating factors may lead to impaired cardiac function, potentially leading to an episode of acute HF. Detection and treatment of precipitating factors plays an important role in patient management. Precipitating factors include excessive salt intake, lack of adherence to medication and diet, myocardial infarction, pulmonary embolism, uncontrolled hypertension, cardiac arrhythmias, valvular disease, cardiotoxic chemotherapy, infection, hypothyroidism, hyperthyroidism, renal dysfunction, and alcohol and drug abuse.[9]
The most common comorbid chronic conditions are hypertension, ischemic heart disease, and hyperlipidemia.[7][9]
[Figure caption and citation for the preceding image starts]: Prevalence of individual chronic conditions in heart failure patients with preserved and reduced ejection fraction. Left panel, men; right panel, womenChamberlain AM, et al. Am J Med. 2015 Jan; 128(1): 38-45; used with permission [Citation ends].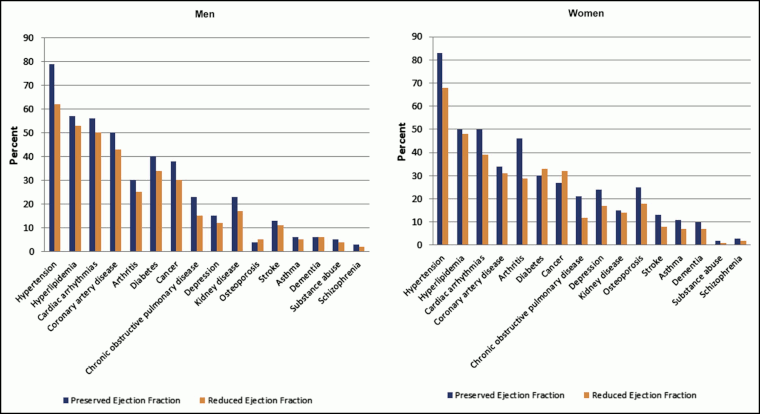
Patient history
HF is primarily a condition of older people.[2][14] The complexity and variety of potential causative factors means that a multitude of patient historic factors may be relevant. Comorbidity is common with 98% to 99% HF patients having at least one comorbid condition (average 3 to 4 comorbidities).[8] A history of hypertension; diabetes mellitus; dyslipidemia; tobacco use; coronary, valvular, or peripheral vascular disease; rheumatic fever; heart murmur or congenital heart disease; personal or family history of myopathy; mediastinal irradiation; and sleep-disturbed breathing should be enquired about. The drug history should record the past or current use of illicit drugs, alcohol, ephedra, or antineoplastic agents such as anthracyclines, trastuzumab, or high-dose cyclophosphamide, because HF may occur years after exposure to these chemotherapy agents. The history and physical evaluation should include specific consideration of noncardiac diseases such as collagen vascular disease, bacterial or parasitic infection, obesity, thyroid excess or deficiency, amyloidosis, carcinoid, and pheochromocytoma.
A detailed family history should be obtained, not only to determine whether there is a familial predisposition to atherosclerotic disease but also to identify relatives with cardiomyopathy, sudden unexplained death, conduction system disease, and skeletal myopathies.
Dyspnea on exertion or at rest is the most common symptom of left-sided HF. With increasing failure, the patient may develop leg edema, weight gain, and abdominal distension due to ascites.[101] Patients with primarily right-sided HF may present with fatigue, leg edema, weight gain, and rarely with abdominal pain due to liver congestion. In late stages they may have abdominal distension due to ascites.
Physical exam
Particular attention should be paid to the cardinal signs of HF. Their presence (and degree) may depend on severity of HF and associated comorbid disease.
General examination may reveal tachycardia and cyanosis. A focused cardiovascular examination may reveal elevated jugular venous pressure, ankle edema, and a displaced apex beat, which suggests cardiomegaly. On auscultation, besides presence of pulmonary rales or crepitation, an S3 gallop may be present, which has prognostic significance.
Auscultation sounds: Third heart sound gallop
Precipitating factors and comorbidity
Clinical manifestations of the underlying etiology of HF may be present, for example:
macroglossia and neuropathy (which may point to infiltrative cardiomyopathy such as amyloidosis)
pallor (which may reflect anemia)
irregularly irregular pulse (reflecting atrial fibrillation)
systolic murmur of aortic stenosis and mid-diastolic murmur of mitral stenosis
overt signs of thyrotoxicosis.
In patients on dialysis, a large arteriovenous fistula may occasionally be the precipitating factor. However, further discussion of the clinical features of all the etiologic conditions is beyond the scope of this topic.
Initial investigations
For all patients, initial investigations should include ECG, chest x-ray, transthoracic echocardiogram, and baseline hematology and blood chemistry, including complete blood count, urinalysis, serum electrolytes (including calcium and magnesium), blood urea nitrogen and creatinine, liver function tests, B-type natriuretic peptide/N-terminal pro-brain natriuretic peptide levels, thyroid function, blood glucose, and lipids.[7][9]
Anemia and high lymphocyte percentage are strong risk factors and prognostic markers of poor survival
In patients presenting with dyspnea, measurement of natriuretic peptide biomarkers is useful to support a diagnosis or exclude HF. However, elevated plasma levels of natriuretic peptides can occur with a wide variety of cardiac and noncardiac causes; therefore, clinical judgment is necessary.
Measurement of left ventricular ejection fraction (LVEF), usually by echocardiogram, is required for classification of HF:[1]
HF with reduced EF (HFrEF): HF with LVEF ≤40%
HF with mildly reduced EF (HFmrEF): HF with LVEF 41% to 49%
HF with preserved EF (HFpEF): HF with LVEF ≥50%
HF with improved EF (HFimpEF): HF with a baseline LVEF of ≤40%, a ≥10-point increase from baseline LVEF, and a second measurement of LVEF of >40%
Initial investigations for common comorbidities: blood glucose, thyroid function tests, and blood lipids are useful to assess for commonly associated comorbid disease. ECG may show evidence of underlying coronary artery disease (CAD). Transthoracic echo can identify valvular, myocardial, or pericardial disease or may reveal evidence of underlying CAD (regional wall motion/thickness abnormalities). A hypertrophied heart may be due to hypertension, hypertrophic obstructive cardiomyopathy, or infiltrative disease such as amyloidosis.
Subsequent investigations
Investigations that help in assessing severity of HF and functional status include noninvasive stress imaging (cardiovascular magnetic resonance imaging [MRI], stress echocardiogram, single photon emission computed tomography [SPECT], positron emission tomography [PET]), standard exercise stress testing (bicycle or treadmill), coronary angiogram, cardiac computed tomography (CT) angiography, cardiopulmonary exercise testing with VO₂ max, 6-minute walking test exercise, and left and right heart catheterization. Troponin measurement is helpful in further risk stratification in chronic HF, as an elevated level is associated with progressive left ventricular dysfunction and increased mortality.[102] Soluble ST2 and galectin-3 (biomarkers for myocardial fibrosis) are predictive of death and hospitalization in patients with HF and are additive to natriuretic peptide in their prognostic value.[103]
Subsequent investigations for comorbidities: based on clinical history, HIV screening and measurement of iron levels and fasting transferrin saturation to screen for hemochromatosis may also be performed. A cardiac MRI scan is particularly useful in the investigation of myocarditis and infiltrative cardiomyopathy. Multi-slice CT (MSCT) can be used for left ventricular ejection fraction (LVEF) estimation. There appears to be no significant difference in LVEF estimation between MSCT and MRI, and also between MSCT and transthoracic echocardiogram.[104] MSCT may offer additional benefit as it provides a combined evaluation of LVEF and coronary artery disease. Endomyocardial biopsy is ordered if acute myocarditis (giant cell or eosinophilic) or primary infiltrative diseases of the heart (amyloidosis, active cardiac sarcoidosis) is suspected.
Other investigations for comorbidities and causes
Following history, physical exam and initial investigations, any suspected comorbidities should be further investigated. See separate topics for details.
Use of this content is subject to our disclaimer
