Most cases of invasive aspergillosis (IA) occur in patients with underlying immune deficiency. Close attention should be paid to the immune status of the patient. Patients at high risk of IA are those with severe, prolonged (>10 days) neutropenia, and allogeneic stem cell recipients with acute or chronic graft-versus-host disease (GVHD).
IA should also be considered in:
Solid organ transplant recipients (particularly in lung and/or heart recipients)
Patients with chronic granulomatous disease (CGD)
Patients receiving high-dose corticosteroids or other immunosuppressive drugs
Patients with poorly controlled diabetes mellitus
Patients with primary immunodeficiency disorders.
The lack of specific clinical features impedes diagnosis. Early diagnosis is paramount to reduce mortality and morbidity. In the high-risk patient with clinical signs and symptoms suspicious of IA, a high resolution computed tomography (CT)/magnetic resonance imaging (MRI) scan, biomarkers (e.g., Aspergillus galactomannan, serum beta-D-glucan), sputum examination, bronchoalveolar lavage (BAL) fluid examination, and tissue biopsy for histopathology and culture of microorganism are helpful in the diagnosis.
Molecular tests such as polymerase chain reaction (PCR) testing of BAL fluid and/or tissue specimen are useful in the early diagnosis of IA.[66]Cruciani M, Mengoli C, Barnes R, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev. 2019 Sep 3;9:CD009551.
https://www.doi.org/10.1002/14651858.CD009551.pub4
http://www.ncbi.nlm.nih.gov/pubmed/31478559?tool=bestpractice.com
[  ]
What is the accuracy of polymerase chain reaction (PCR) blood testing for the diagnosis of invasive aspergillosis in immunocompromised people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2775/fullShow me the answer
]
What is the accuracy of polymerase chain reaction (PCR) blood testing for the diagnosis of invasive aspergillosis in immunocompromised people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2775/fullShow me the answer
Chronic pulmonary aspergillosis (CPA) should be considered in patients with chronic lung disease and radiographs showing intracavitary mass lesions. These are usually incidental findings on a routine chest x-ray (CXR) or during evaluation of hemoptysis. Guidelines suggest that diagnosis of CPA requires imaging, direct evidence of Aspergillus infection or an immunologic response to Aspergillus and exclusion of other diagnoses, with disease present for at least 3 months.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
See Diagnostic criteria.
Clinical manifestations
Invasive pulmonary aspergillosis presents with fever, mild to moderate nonproductive cough, and pleuritic chest pain. Pleuritic chest pain in a neutropenic patient or in a stem cell recipient with GVHD should raise strong suspicion of IA. Hemoptysis may be present and may suggest the presence of a lung lesion eroding into a neighboring blood vessel. Catastrophic hemoptysis may occur, particularly with recovery of neutrophils after chemotherapy. Dyspnea may be present, suggesting extensive lung involvement, and may be seen with rejection of a transplanted lung.
Invasive sinus disease may present with headache, congestion, facial pain with or without sinus drainage, or sinus tenderness. Concomitant involvement of sinus and lungs may occur.
Extension of sinus disease into the eye/brain may lead to proptosis, cranial nerve palsies, altered mental status, and seizures.
Skin involvement is not uncommon in IA. Single or multiple discrete, erythematous, mildly tender nodules of varying sizes with a necrotic and often ulcerated center (ecthyma gangrenosum) are mostly seen in immunocompromised patients. They may occur in disseminated disease or local invasion after trauma. Occasionally, burns or surgical wounds may be infected with Aspergillus.
Symptoms of CPA include a chronic cough, breathlessness, chest discomfort, weight loss, and malaise.[4]Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015 Mar;70(3):270-7.
https://thorax.bmj.com/content/70/3/270.long
http://www.ncbi.nlm.nih.gov/pubmed/25354514?tool=bestpractice.com
Simple aspergilloma is mostly asymptomatic. It can present as a self-limiting mild hemoptysis; however, severe hemoptysis may occur in a minority of cases.
Imaging
Pulmonary invasive aspergillosis (IA)
CXR may reveal nodules, consolidation, or frequently nonspecific infiltrates. Often CXR shows no abnormalities. If index of suspicion is high, chest CT scan should be obtained.
High-resolution CT scan of chest is the preferred radiologic method as it is useful in detecting early lesions suggestive of pulmonary aspergillosis. The scan may show single or multiple nodules scattered over 1 or both lungs, generally in the periphery of the lung fields. Smaller nodules (<1 cm), ground-glass opacities, and consolidation are nonspecific features and do not necessarily suggest pulmonary IA.[67]Escuissato D, Gasparetto EL, Marchiori E, et al. Pulmonary infections after bone marrow transplantation: high-resolution CT findings in 111 patients. AJR Am J Roentgenol. 2005 Sep;185(3):608-15.
http://www.ajronline.org/doi/full/10.2214/ajr.185.3.01850608
http://www.ncbi.nlm.nih.gov/pubmed/16120907?tool=bestpractice.com
The presence of macronodules (1 cm or larger) in a high-risk patient is highly suggestive of IA, and may be seen in other conditions including other invasive fungal infections, tuberculosis, nocardiosis, and bacterial infections.[67]Escuissato D, Gasparetto EL, Marchiori E, et al. Pulmonary infections after bone marrow transplantation: high-resolution CT findings in 111 patients. AJR Am J Roentgenol. 2005 Sep;185(3):608-15.
http://www.ajronline.org/doi/full/10.2214/ajr.185.3.01850608
http://www.ncbi.nlm.nih.gov/pubmed/16120907?tool=bestpractice.com
In the leukemic patient with neutropenia, early disease is characterized by a haziness representing hemorrhage/edema surrounding the nodules ("halo sign").[68]Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001 Jan 1;19(1):253-9.
http://www.ncbi.nlm.nih.gov/pubmed/11134220?tool=bestpractice.com
With clinical improvement (e.g., reversal of underlying immune deficiency), the halo sign may disappear. The "air-crescent sign" can be observed instead. It is indicative of a necrotic lesion contracting from viable lung tissue, creating a cavity within where the air is trapped. The halo sign is indicative of early disease and thus is useful in early diagnosis, while the air-crescent sign indicates that the disease has been present for >6 to 7 days. Therapy initiated in patients with halo sign is associated with improved outcome.[69]Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007 Feb 1;44(3):373-9.
http://cid.oxfordjournals.org/content/44/3/373.long
http://www.ncbi.nlm.nih.gov/pubmed/17205443?tool=bestpractice.com
During therapy, the nodular lesions initially enlarge, suggesting that the process may be worsening. After about 7 days of therapy, however, CT scans show improvement. Pulmonary macronodules, the halo sign, and the air-crescent sign have been best studied in patients with IA and hematologic malignancy or stem cell transplantation. Radiologic features are not as well characterized in other settings with IA.
Invasive aspergillosis at other sites (e.g., skin, brain, sinuses)
IA suspected at other sites such as sinuses and brain may also be evaluated with CT scan or MRI. X-rays of the sinuses are not helpful. CT is the preferred imaging modality.[68]Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001 Jan 1;19(1):253-9.
http://www.ncbi.nlm.nih.gov/pubmed/11134220?tool=bestpractice.com
[67]Escuissato D, Gasparetto EL, Marchiori E, et al. Pulmonary infections after bone marrow transplantation: high-resolution CT findings in 111 patients. AJR Am J Roentgenol. 2005 Sep;185(3):608-15.
http://www.ajronline.org/doi/full/10.2214/ajr.185.3.01850608
http://www.ncbi.nlm.nih.gov/pubmed/16120907?tool=bestpractice.com
With sinus disease, in addition to the opacity/mass within the sinus cavity, bone erosion of the surrounding sinus walls is highly suggestive of an aggressive infection. In brain disease, space-occupying lesions with surrounding edema, abscesses, and hemorrhage can be seen.
CPA
CXR may reveal one or more lung cavities with or without aspergilloma, infiltrates, nodules, pleural thickening, parenchymal damage, and fibrosis.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
[4]Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015 Mar;70(3):270-7.
https://thorax.bmj.com/content/70/3/270.long
http://www.ncbi.nlm.nih.gov/pubmed/25354514?tool=bestpractice.com
[70]Zhong H, Wang Y, Gu Y, et al. Clinical features, diagnostic test performance, and prognosis in different subtypes of chronic pulmonary aspergillosis. Front Med (Lausanne). 2022 Feb 11:9:811807.
https://www.frontiersin.org/articles/10.3389/fmed.2022.811807/full
http://www.ncbi.nlm.nih.gov/pubmed/35223906?tool=bestpractice.com
X-ray is the initial imaging method, but CT scan can provide better definition and location of findings.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
Aspergilloma
Single upper lobe lesions are the most common finding on CXR. Multiple lesions are rarely seen. An upper lobe, mobile, intracavitary mass with an air-crescent in the periphery (Monod's sign) is strongly suggestive of aspergilloma. Plain x-rays are usually adequate. Occasionally chest CT is required. In radiographs a change in the position of the fungal ball may be seen with a change in the position of the patient.[71]Roberts CM, Citron KM, Strickland B. Intrathoracic aspergilloma: role of CT in diagnosis and treatment. Radiology. 1987 Oct;165(1):123-8.
http://www.ncbi.nlm.nih.gov/pubmed/3628758?tool=bestpractice.com
Periodic CXRs are adequate for the follow-up of asymptomatic aspergilloma.
[Figure caption and citation for the preceding image starts]: "Halo" sign in early pulmonary aspergillosisFrom the collection of Dr P. Chandrasekar; used with permission [Citation ends].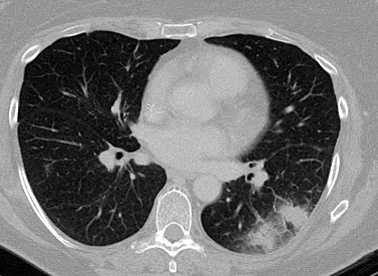 [Figure caption and citation for the preceding image starts]: "Air-crescent" sign in late pulmonary aspergillosisFrom the collection of Dr P. Chandrasekar; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: "Air-crescent" sign in late pulmonary aspergillosisFrom the collection of Dr P. Chandrasekar; used with permission [Citation ends].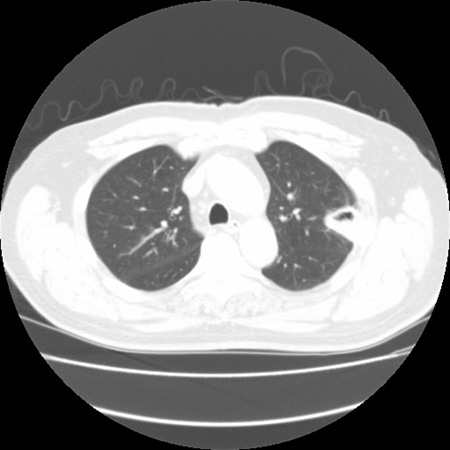
Serology/bronchoalveolar lavage (BAL)
Invasive aspergillosis
The diagnosis of IA has remained a challenge due to the nonspecific clinical presentation of IA, the low sensitivity of microscopy and culture of lower respiratory specimens, and the difficulty of obtaining tissue for histopathology in critically ill patients. As a result, biomarkers such as Aspergillus galactomannan (GM) antigen and serum beta-D-glucan have been evaluated, mostly in stem cell recipients and leukemic patients with neutropenia.[72]Wheat LJ, Walsh TJ. Diagnosis of invasive aspergillosis by galactomannan antigenemia detection using an enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 2008 Apr;27(4):245-51.
http://www.ncbi.nlm.nih.gov/pubmed/18193305?tool=bestpractice.com
[73]Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005 Sep 1;41(5):654-9.
http://www.ncbi.nlm.nih.gov/pubmed/16080087?tool=bestpractice.com
[74]Odabasi Z, Mattiuzzi G, Estey E, et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004 Jul 15;39(2):199-205.
http://cid.oxfordjournals.org/content/39/2/199.long
http://www.ncbi.nlm.nih.gov/pubmed/15307029?tool=bestpractice.com
Galactomannan (GM) antigen
GM antigen is a polysaccharide cell wall component of Aspergillus species that is released into the systemic circulation during fungal growth in tissue.
A double-sandwich enzyme-linked immunosorbent assay (ELISA) method exists for detection of GM (optical density index 0.5 or greater on 2 occasions is positive).[75]Marr KA, Balajee SA, McLaughlin L, et al. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J Infect Dis. 2004 Aug 1;190(3):641-9.
http://jid.oxfordjournals.org/content/190/3/641.long
http://www.ncbi.nlm.nih.gov/pubmed/15243943?tool=bestpractice.com
[76]Maertens JA, Klont R, Masson C, et al. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin Infect Dis. 2007 May 15;44(10):1329-36.
http://cid.oxfordjournals.org/content/44/10/1329.long
http://www.ncbi.nlm.nih.gov/pubmed/17443470?tool=bestpractice.com
The combination of a high-risk patient with suggestive clinical and radiologic (CT scan) findings and a positive serum GM may be considered adequate for a diagnosis of "probable" IA, thus avoiding invasive procedures such as tissue (lung) biopsy.[77]Maertens J, Van Eldere J, Verhaegen J, et al. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002 Nov 1;186(9):1297-306.
http://jid.oxfordjournals.org/content/186/9/1297.long
http://www.ncbi.nlm.nih.gov/pubmed/12402199?tool=bestpractice.com
[78]Maertens J, Verhaegen J, Lagrou K, et al. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood. 2001 Mar 15;97(6):1604-10.
https://ashpublications.org/blood/article/97/6/1604/107293/Screening-for-circulating-galactomannan-as-a
http://www.ncbi.nlm.nih.gov/pubmed/11238098?tool=bestpractice.com
The sensitivity and specificity of serial serum GM is 67% to 100% in acute leukemia patients and 86% to 98% in stem cell recipients, respectively.[76]Maertens JA, Klont R, Masson C, et al. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin Infect Dis. 2007 May 15;44(10):1329-36.
http://cid.oxfordjournals.org/content/44/10/1329.long
http://www.ncbi.nlm.nih.gov/pubmed/17443470?tool=bestpractice.com
[77]Maertens J, Van Eldere J, Verhaegen J, et al. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002 Nov 1;186(9):1297-306.
http://jid.oxfordjournals.org/content/186/9/1297.long
http://www.ncbi.nlm.nih.gov/pubmed/12402199?tool=bestpractice.com
Serial monitoring of GM has detected IA between 6 and 14 days earlier than radiographic findings.[79]Sulahian A, Boutboul F, Ribaud P, et al. Value of antigen detection using an enzyme immunoassay in the diagnosis and prediction of invasive aspergillosis in two adult and pediatric hematology units during a 4-year prospective study. Cancer. 2001 Jan 15;91(2):311-8.
http://www.ncbi.nlm.nih.gov/pubmed/11180076?tool=bestpractice.com
False-positive results are seen with other fungi such as Histoplasma, Blastomyces, Geotrichum, and Penicillium species, and bacteria (e.g., Bifidobacterium).[72]Wheat LJ, Walsh TJ. Diagnosis of invasive aspergillosis by galactomannan antigenemia detection using an enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 2008 Apr;27(4):245-51.
http://www.ncbi.nlm.nih.gov/pubmed/18193305?tool=bestpractice.com
[80]Mennink-Kersten MA, Klont RR, Warris A, et al. Bifidobacterium lipoteichoic acid and false ELISA reactivity in Aspergillus antigen detection. Lancet. 2004 Jan 24;363(9405):325-7.
http://www.ncbi.nlm.nih.gov/pubmed/14751710?tool=bestpractice.com
The use of beta-lactam antibiotics such as piperacillin-tazobactam and amoxicillin-clavulanic acid may show false-positive results.[81]Sulahian A, Touratier S, Ribaud P. False positive test for Aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N Engl J Med. 2003 Dec 11;349(24):2366-7.
http://www.ncbi.nlm.nih.gov/pubmed/14668472?tool=bestpractice.com
In the presence of mold-active drugs used as prophylaxis or therapy, the sensitivity of GM assay is reduced. The sensitivity may be lower in non-neutropenic patients, possibly due to a lower fungal burden.
The combined use of serum GM antigen assay and chest CT improves the detection of pulmonary IA, permitting earlier initiation of therapy.[78]Maertens J, Verhaegen J, Lagrou K, et al. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood. 2001 Mar 15;97(6):1604-10.
https://ashpublications.org/blood/article/97/6/1604/107293/Screening-for-circulating-galactomannan-as-a
http://www.ncbi.nlm.nih.gov/pubmed/11238098?tool=bestpractice.com
Data suggest that GM antigen measurements in BAL fluid are more sensitive than serum GM and have a better predictive value, and BAL GM antigen test has now become an accepted method for diagnosis.[82]Musher B, Fredricks D, Leisenring W, et al. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J Clin Microbiol. 2004 Dec;42(12):5517-22.
http://jcm.asm.org/cgi/content/full/42/12/5517?view=long&pmid=15583275
http://www.ncbi.nlm.nih.gov/pubmed/15583275?tool=bestpractice.com
[83]Meersseman W, Lagrou K, Maertens J, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med. 2008 Jan 1;177(1):27-34.
http://www.atsjournals.org/doi/pdf/10.1164/rccm.200704-606OC
http://www.ncbi.nlm.nih.gov/pubmed/17885264?tool=bestpractice.com
BAL fluid GM antigen measurement of 1.5 optical density index or higher appears to be a strong predictor of IA in immunocompromised patients (specificity >90%).[84]de Heer K, Gerritsen MG, Visser CE, et al. Galactomannan detection in broncho-alveolar lavage fluid for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2019 May 20;5:CD012399.
https://www.doi.org/10.1002/14651858.CD012399.pub2
http://www.ncbi.nlm.nih.gov/pubmed/31107543?tool=bestpractice.com
Serum (1-3)-beta-D-glucan
(1-3)-beta-D-glucan is a component in the cell wall of many fungi (with the exception of Zygomycetes and Cryptococcus), and a serologic diagnostic method for invasive fungi.[73]Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005 Sep 1;41(5):654-9.
http://www.ncbi.nlm.nih.gov/pubmed/16080087?tool=bestpractice.com
[74]Odabasi Z, Mattiuzzi G, Estey E, et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis. 2004 Jul 15;39(2):199-205.
http://cid.oxfordjournals.org/content/39/2/199.long
http://www.ncbi.nlm.nih.gov/pubmed/15307029?tool=bestpractice.com
This test is a variation of the limulus assay used to detect endotoxin. The presence of serum glucan is not specific for Aspergillus and false-positive results can occur due to blood collection tubes, gauze, and contaminated membrane filters.
One Cochrane review found wide variation in sensitivity and specificity of commercially available tests for serum (1-3)-beta-D-glucan in detecting selected invasive fungal infections, including aspergillosis. Sensitivity ranged from 27% to 100%, and specificity ranged from 0% to 100%; therefore, accuracy of diagnosis could not be determined.[85]White SK, Schmidt RL, Walker BS, et al. (1→3)-β-D-glucan testing for the detection of invasive fungal infections in immunocompromised or critically ill people. Cochrane Database Syst Rev. 2020 Jul 21;(7):CD009833.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009833.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/32693433?tool=bestpractice.com
[  ]
For immunocompromised or critically ill people, what is the accuracy of D‐glucan testing for detection of invasive fungal infection?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3291/fullShow me the answer
]
For immunocompromised or critically ill people, what is the accuracy of D‐glucan testing for detection of invasive fungal infection?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3291/fullShow me the answer
Polymerase chain reaction (PCR)
Polymerase chain reaction diagnosis, based on amplification of Aspergillus-specific fungal genes (usually ribosomal DNA) in blood and BAL fluid, has shown considerable promise for early diagnosis. In BAL fluid, two positive PCR test results have a higher positive predictive value to rule in IA.[66]Cruciani M, Mengoli C, Barnes R, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev. 2019 Sep 3;9:CD009551.
https://www.doi.org/10.1002/14651858.CD009551.pub4
http://www.ncbi.nlm.nih.gov/pubmed/31478559?tool=bestpractice.com
[  ]
What is the accuracy of polymerase chain reaction (PCR) blood testing for the diagnosis of invasive aspergillosis in immunocompromised people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2775/fullShow me the answer PCR testing in histopathology specimens also increases the diagnostic yield.[66]Cruciani M, Mengoli C, Barnes R, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev. 2019 Sep 3;9:CD009551.
https://www.doi.org/10.1002/14651858.CD009551.pub4
http://www.ncbi.nlm.nih.gov/pubmed/31478559?tool=bestpractice.com
PCR-based test results may be falsely positive because of ubiquitous presence of Aspergillusconidia. Combining the PCR-based test with other noninvasive nonculture-based diagnostic methods (i.e., serum GM test and serum beta-D-glucan assay) is an important area for early diagnosis of invasive aspergillosis.
]
What is the accuracy of polymerase chain reaction (PCR) blood testing for the diagnosis of invasive aspergillosis in immunocompromised people?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2775/fullShow me the answer PCR testing in histopathology specimens also increases the diagnostic yield.[66]Cruciani M, Mengoli C, Barnes R, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev. 2019 Sep 3;9:CD009551.
https://www.doi.org/10.1002/14651858.CD009551.pub4
http://www.ncbi.nlm.nih.gov/pubmed/31478559?tool=bestpractice.com
PCR-based test results may be falsely positive because of ubiquitous presence of Aspergillusconidia. Combining the PCR-based test with other noninvasive nonculture-based diagnostic methods (i.e., serum GM test and serum beta-D-glucan assay) is an important area for early diagnosis of invasive aspergillosis.
CPA
In patients with imaging and history suggestive of CPA, diagnosis can be confirmed with serum Aspergillus immunoglobulin G (IgG) or precipitins, or Aspergillus antigen or DNA in respiratory fluids.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
Aspergillus antibodies
Aspergillus IgG antibody test is the most sensitive test for chronic cavitary pulmonary aspergillosis (CCPA).[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
Serum IgG antibodies to Aspergillus or precipitins are positive in most patients with CPA.
False-negative cases may be seen in patients receiving corticosteroid therapy or in those with aspergilloma due to species other than A fumigatus.[86]Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest. 2002 Jun;121(6):1988-99.
http://www.ncbi.nlm.nih.gov/pubmed/12065367?tool=bestpractice.com
GM antigen
When used in the diagnosis of CPA, BAL fluid should be used (not serum). Specificity and sensitivity is lower than Aspergillus antibody tests.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
PCR
Sensitivity of PCR testing of respiratory secretions is lower than antibody testing, but higher than culture.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[87]Wilopo BAP, Richardson MD, Denning DW. Diagnostic aspects of chronic pulmonary aspergillosis: present and new directions. Curr Fungal Infect Rep. 2019 Nov 25;13:292-300.
https://link.springer.com/article/10.1007/s12281-019-00361-7
Microbiology
Pulmonary invasive aspergillosis (IA)
Cough is generally nonproductive in these patients. Sputum, when available, is usually negative by fungal stain and culture. A positive finding is highly significant in a high-risk patient (immunocompromised).[11]Perfect JR, Cox GM, Lee JY, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001 Dec 1;33(11):1824-33.
https://academic.oup.com/cid/article-lookup/doi/10.1086/322606
http://www.ncbi.nlm.nih.gov/pubmed/11692293?tool=bestpractice.com
However, in a low-risk patient (immunocompetent), Aspergillus in sputum may simply represent colonization needing no further intervention.[11]Perfect JR, Cox GM, Lee JY, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001 Dec 1;33(11):1824-33.
https://academic.oup.com/cid/article-lookup/doi/10.1086/322606
http://www.ncbi.nlm.nih.gov/pubmed/11692293?tool=bestpractice.com
[88]Horvath JA, Dummer S. The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med. 1996 Feb;100(2):171-8.
http://www.ncbi.nlm.nih.gov/pubmed/8629651?tool=bestpractice.com
Aspergillus species grow well on standard media and can be identified to a species level in most laboratories. Culture from a sterile site is diagnostic of IA. Blood cultures are usually negative even in disseminated cases.
Commonly used invasive diagnostic procedures are:
Bronchoscopy with BAL and/or biopsy
Percutaneous transthoracic CT-guided needle aspiration
Video-assisted thoracoscopic biopsy.
Specimens obtained may show characteristic angular, dichotomously branching, septate hyphae, and Aspergillus species in culture. Culture confirmation is critical to distinguish Aspergillus from other fungi with similar morphologic features, such as Fusarium and Scedosporium.[17]Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005 Oct;5(10):609-22.
http://www.ncbi.nlm.nih.gov/pubmed/16183515?tool=bestpractice.com
False-negative results occur with specimens obtained from unaffected areas, with inadequate specimens, and in patients already receiving antifungal therapy. Thus, lack of positive fungal smear or culture does not rule out the diagnosis of IA. Also, invasive procedures may not be possible in critically ill patients or those with thrombocytopenia.
Invasive aspergillosis at other sites (e.g., skin, brain, sinuses)
[Figure caption and citation for the preceding image starts]: Gomori methenamine silver (GMS) of lung tissue showing dichotomously branching, septate hyphae of AspergillusFrom the collection of Dr P. Chandrasekar; used with permission [Citation ends].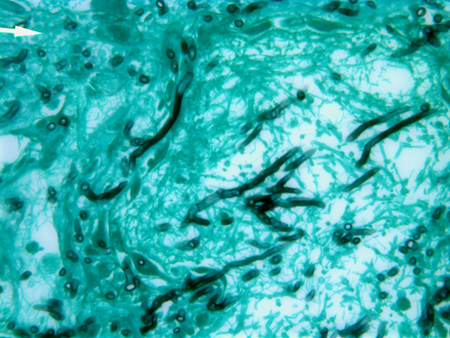
CPA
Direct microscopy or fungal culture of respiratory specimens may identify the presence of Aspergillus. However, culture positivity rates vary widely and results should be interpreted with caution.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
[89]Takazono T, Izumikawa K. Recent advances in diagnosing chronic pulmonary aspergillosis. Front Microbiol. 2018 Aug 17:9:1810.
https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2018.01810/full
http://www.ncbi.nlm.nih.gov/pubmed/30174658?tool=bestpractice.com
Testing multiple samples increases the probability of a positive culture or microscopy test, but the majority of patients have negative sputum cultures.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
Additionally, as Aspergillus are ubiquitous in the environment, their presence in sputum is not necessarily diagnostic.[89]Takazono T, Izumikawa K. Recent advances in diagnosing chronic pulmonary aspergillosis. Front Microbiol. 2018 Aug 17:9:1810.
https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2018.01810/full
http://www.ncbi.nlm.nih.gov/pubmed/30174658?tool=bestpractice.com
Histopathology
Invasive aspergillosis (IA)
Tissue biopsy is the most definitive method of diagnosis. Biopsy may be obtained by:
VATS is the preferred method, as biopsy is obtained under direct vision and is less invasive compared with open lung biopsy. Specimen obtained by a transbronchial or CT-guided approach is generally suboptimal and may be associated with complications such as uncontrolled bleeding or pneumothorax. Thrombocytopenia is common in leukemia and stem cell recipients, making invasive procedures hazardous. Often a biopsy procedure cannot be performed in view of platelet transfusion-refractory thrombocytopenia or severe illness, thus leading clinicians to make empiric treatment choices.
Other biopsy sites may include skin, sinus tissue, brain or, uncommonly, bone, heart, pericardium, or abdominal organs.
Specimens obtained by biopsy must be sent in saline for microbiologic culture and in formalin for pathology. Acute-angle branching, septate, narrow hyphae, with tissue invasion and surrounding inflammatory infiltrates along with necrosis, are findings highly suggestive of IA.[17]Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005 Oct;5(10):609-22.
http://www.ncbi.nlm.nih.gov/pubmed/16183515?tool=bestpractice.com
As Aspergillus is angioinvasive, the organism is frequently found within the vasculature, causing thrombosis, tissue infarction, and coagulative necrosis. Other fungi such as Fusarium and Scedosporium ( Pseudoallescheria) may have similar features requiring culture confirmation of the organism.
CPA
In certain cases, microscopic examination of tissue obtained by biopsy is necessary for diagnosis.
In CCPA, biopsy may show inflammatory cells, fibrosis, granulomata, and hyphae.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
If hyphae are invading the lung parenchyma, then acute or subacute IA is diagnosed.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
Examination of aspergilloma shows fungal mycelia, inflammatory cells, tissue debris, fibrin, and mucus.[4]Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015 Mar;70(3):270-7.
https://thorax.bmj.com/content/70/3/270.long
http://www.ncbi.nlm.nih.gov/pubmed/25354514?tool=bestpractice.com
Aspergillus nodules are diagnosed after excision biopsy, usually following suspicion for malignancy. Single nodules that are completely excised may not need any further treatment.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
[4]Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015 Mar;70(3):270-7.
https://thorax.bmj.com/content/70/3/270.long
http://www.ncbi.nlm.nih.gov/pubmed/25354514?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Diagnostic algorithm for suspected invasive aspergillosisCreated by authors [Citation ends].
 ]
]
 [Figure caption and citation for the preceding image starts]: "Air-crescent" sign in late pulmonary aspergillosisFrom the collection of Dr P. Chandrasekar; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: "Air-crescent" sign in late pulmonary aspergillosisFrom the collection of Dr P. Chandrasekar; used with permission [Citation ends].
 ]
]
 ]
PCR testing in histopathology specimens also increases the diagnostic yield.[66] PCR-based test results may be falsely positive because of ubiquitous presence of Aspergillusconidia. Combining the PCR-based test with other noninvasive nonculture-based diagnostic methods (i.e., serum GM test and serum beta-D-glucan assay) is an important area for early diagnosis of invasive aspergillosis.
]
PCR testing in histopathology specimens also increases the diagnostic yield.[66] PCR-based test results may be falsely positive because of ubiquitous presence of Aspergillusconidia. Combining the PCR-based test with other noninvasive nonculture-based diagnostic methods (i.e., serum GM test and serum beta-D-glucan assay) is an important area for early diagnosis of invasive aspergillosis. 
