The two key elements for successful therapy in patients with invasive aspergillosis (IA) are:
Reversal of the underlying immune deficiency
Early introduction of antifungal therapy.
Antifungal therapy may be definitive, empiric, or preemptive.
Definitive treatment is provided in a setting of confirmed/probable diagnosis.
Empiric treatment is given in a suspected diagnosis in a high-risk patient with clinical features suggestive of the infection without further confirmation (e.g., radiology, serology).
Preemptive therapy is provided in cases of strongly suspected IA in a high-risk patient with suggestive clinical features plus the presence of additional evidence (e.g., suggestive computed tomography [CT] scan and/or positive biomarkers).
In occasional cases, a surgical approach in removing infected tissue may play a critical role for a successful outcome.
Treatment of chronic pulmonary aspergillosis (CPA) depends on the type. Chronic cavitary pulmonary aspergillosis (CCPA) may require antifungal therapy to stabilize progression and improve symptoms. Treatment of simple aspergilloma in asymptomatic patients is not warranted. Good-quality evidence-based data to support the use of antifungal therapy are lacking.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
In symptomatic patients with severe hemoptysis surgical treatment should be considered.
Suspected invasive aspergillosis (possible diagnosis)
In high-risk patients, empiric therapy may be used when the diagnosis of IA is suspected (e.g., in neutropenic patients with fever unresponsive to broad-spectrum antibacterial agents without an obvious focus of infection).[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
Fever may be due to nonfungal etiology. However, since the diagnosis is difficult to confirm, antifungals are frequently employed. Liposomal amphotericin B or an echinocandin are the drugs used in this context.[97]Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002 Jan 24;346(4):225-34.
http://www.ncbi.nlm.nih.gov/pubmed/11807146?tool=bestpractice.com
[98]Freemantle N, Tharmanathan P, Herbrecht R. Systematic review and mixed treatment comparison of randomized evidence for empirical, pre-emptive and directed treatment strategies for invasive mould disease. J Antimicrob Chemother. 2011 Jan;66 Suppl 1:i25-35.
http://jac.oxfordjournals.org/content/66/suppl_1/i25.full.pdf+html
http://www.ncbi.nlm.nih.gov/pubmed/21177401?tool=bestpractice.com
The Infectious Diseases Society of America (IDSA) guidelines also recommend voriconazole.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
Invasive aspergillosis (confirmed/probable diagnosis)
1. Reversal of the underlying immune deficiency
The use of colony-stimulating factors may reduce the duration of neutropenia. Discontinuing or reducing the dose of corticosteroids may help restore immune function. However, in many situations the underlying immunological deficiency may not be correctable (e.g., in presence of severe graft-versus-host disease [GVHD]). In such cases, the prognosis is generally poor. Early diagnosis followed by early initiation of therapy with antifungal agents improves outcome.[99]von Eiff M, Roos N, Schulten R, et al. Pulmonary aspergillosis: early diagnosis improves survival. Respiration. 1995;62(6):341-7.
http://www.ncbi.nlm.nih.gov/pubmed/8552866?tool=bestpractice.com
2. Antifungal therapy
Classes of antifungal drugs with good in vitro/in vivo activity against Aspergillus species include:
Polyenes (e.g., amphotericin B)
Azoles (e.g., voriconazole, posaconazole, isavuconazonium)
Echinocandins (e.g., caspofungin, micafungin).
Isavuconazonium and voriconazole are the drugs of choice in the treatment of confirmed/probable IA.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[98]Freemantle N, Tharmanathan P, Herbrecht R. Systematic review and mixed treatment comparison of randomized evidence for empirical, pre-emptive and directed treatment strategies for invasive mould disease. J Antimicrob Chemother. 2011 Jan;66 Suppl 1:i25-35.
http://jac.oxfordjournals.org/content/66/suppl_1/i25.full.pdf+html
http://www.ncbi.nlm.nih.gov/pubmed/21177401?tool=bestpractice.com
[100]Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002 Mar 1;34(5):563-71.
http://cid.oxfordjournals.org/content/34/5/563.long
http://www.ncbi.nlm.nih.gov/pubmed/11807679?tool=bestpractice.com
Although efficacies of isavuconazonium and voriconazole are similar, the former appears to have a better safety profile.[16]Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018 May;24 Suppl 1:e1-e38.
https://www.doi.org/10.1016/j.cmi.2018.01.002
http://www.ncbi.nlm.nih.gov/pubmed/29544767?tool=bestpractice.com
Isavuconazonium is a prodrug of isavuconazole, a broad-spectrum antifungal agent with activity against both Aspergillus and Mucor. It is indicated for the treatment of adults with IA.[101]Bates DW, Su L, Yu DT, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001 Mar 1;32(5):686-93.
https://academic.oup.com/cid/article/32/5/686/357901
http://www.ncbi.nlm.nih.gov/pubmed/11229835?tool=bestpractice.com
Isavuconazole is only available as isavuconazonium in the US.
Voriconazole has shown to be safer and more effective than conventional amphotericin B deoxycholate.[102]Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002 Aug 8;347(6):408-15.
http://www.nejm.org/doi/full/10.1056/NEJMoa020191#t=article
http://www.ncbi.nlm.nih.gov/pubmed/12167683?tool=bestpractice.com
However, it has not been compared with any lipid formulation of amphotericin B.
There is a well established link between voriconazole and cutaneous reactions. This includes phototoxicity and importantly, cutaneous squamous cell carcinoma, which has been described as aggressive and multifocal, in multiple case reports.[103]Williams K, Mansh M, Chin-Hong P, et al. Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis. 2014 Apr;58(7):997-1002.
https://academic.oup.com/cid/article/58/7/997/412197
http://www.ncbi.nlm.nih.gov/pubmed/24363331?tool=bestpractice.com
Such reactions have been widely described in 1% to 2% of patients receiving >12 weeks of therapy.[104]Clancy CJ, Nguyen MH. Long-term voriconazole and skin cancer: is there cause for concern? Curr Infect Dis Rep. 2011 Dec;13(6):536-43.
http://www.ncbi.nlm.nih.gov/pubmed/21997681?tool=bestpractice.com
[105]Miller DD, Cowen EW, Nguyen JC, et al. Melanoma associated with long-term voriconazole therapy: a new manifestation of chronic photosensitivity. Arch Dermatol. 2010 Mar;146(3):300-4.
https://jamanetwork.com/journals/jamadermatology/fullarticle/209667
http://www.ncbi.nlm.nih.gov/pubmed/20083676?tool=bestpractice.com
Photosensitivity induced by voriconazole results in a sunburn-like erythema that is limited to sun-exposed sites.[103]Williams K, Mansh M, Chin-Hong P, et al. Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis. 2014 Apr;58(7):997-1002.
https://academic.oup.com/cid/article/58/7/997/412197
http://www.ncbi.nlm.nih.gov/pubmed/24363331?tool=bestpractice.com
The underlying mechanisms for this are unclear and establishing a definitive causative link between voriconazole and squamous cell carcinoma will require further studies. In patients requiring longer duration treatment with voriconazole, patient education and careful examination of skin should be regularly performed alongside advice to avoid excess sun exposure and to use ultraviolet protection liberally.
Alternative options to isavuconazonium or voriconazole are posaconazole or a lipid formulation of amphotericin B, either amphotericin B lipid complex or liposomal amphotericin B.[106]Chandrasekar PH, Ito JI. Amphotericin B lipid complex in the management of invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2005 May 1;40 Suppl 6:S392-400.
https://academic.oup.com/cid/article/40/Supplement_6/S392/273030
http://www.ncbi.nlm.nih.gov/pubmed/15809925?tool=bestpractice.com
[107]Walsh TJ, Hiemenz JW, Seibel NL, et al. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998 Jun;26(6):1383-96.
http://www.ncbi.nlm.nih.gov/pubmed/9636868?tool=bestpractice.com
[108]Cornely OA, Maertens J, Bresnik M, et al.; AmBiLoad Trial Study Group. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-load regimen with standard dosing (AmBiLoad trial). Clin Infect Dis. 2007 May 15;44(10):1289-97.
https://academic.oup.com/cid/article/44/10/1289/355162
http://www.ncbi.nlm.nih.gov/pubmed/17443465?tool=bestpractice.com
Posaconazole demonstrated noninferiority to voriconazole in the treatment of invasive aspergillosis in one randomized controlled trial; posaconazole was also associated with fewer treatment-related adverse events.[109]Maertens JA, Rahav G, Lee DG, et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet. 2021 Feb 6;397(10273):499-509.
http://www.ncbi.nlm.nih.gov/pubmed/33549194?tool=bestpractice.com
In areas of known and increasing azole-resistance, a lipid formulation of amphotericin B should be considered the first-line agent until the results of resistance testing are available.[16]Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018 May;24 Suppl 1:e1-e38.
https://www.doi.org/10.1016/j.cmi.2018.01.002
http://www.ncbi.nlm.nih.gov/pubmed/29544767?tool=bestpractice.com
In view of the nephrotoxic potential, conventional amphotericin B is no longer favored.[101]Bates DW, Su L, Yu DT, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001 Mar 1;32(5):686-93.
https://academic.oup.com/cid/article/32/5/686/357901
http://www.ncbi.nlm.nih.gov/pubmed/11229835?tool=bestpractice.com
IA due to Aspergillus terreus may not respond to amphotericin B.[19]Walsh TJ, Petraitis V, Petraitiene R, et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis. 2003 Jul 15;188(2):305-19.
http://jid.oxfordjournals.org/content/188/2/305.long
http://www.ncbi.nlm.nih.gov/pubmed/12854088?tool=bestpractice.com
[20]Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7(1):31-43.
http://www.ncbi.nlm.nih.gov/pubmed/16489841?tool=bestpractice.com
Patients generally show clinical/radiologic improvement in 5 to 7 days. Improvement is enhanced if the underlying immune deficiency is corrected - typically, the return of neutrophils. If the immune system remains impaired, the outcome is generally poor.
Therapeutic drug monitoring (TDM) is useful in the management of IA with voriconazole, isavuconazonium, and posaconazole where available. In case of failure of therapy with voriconazole, isavuconazonium, or lipid formulations of amphotericin B, clinical deterioration is apparent in 7 to 10 days. Additional measures include checking serum concentrations of voriconazole, switching from voriconazole to lipid formulations of amphotericin B and/or an echinocandin, or adding an echinocandin to therapy with voriconazole for potential synergy. Combination therapy (an azole antifungal plus an echinocandin) may be more effective than monotherapy with an azole.[110]Marr KA, Boeckh M, Carter RA, et al. Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis. 2004 Sep 15;39(6):797-802.
http://cid.oxfordjournals.org/content/39/6/797.long
http://www.ncbi.nlm.nih.gov/pubmed/15472810?tool=bestpractice.com
[111]Singh N, Limaye AP, Forrest G, et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation. 2006 Feb 15;81(3):320-6.
http://www.ncbi.nlm.nih.gov/pubmed/16477215?tool=bestpractice.com
In critically ill patients, combination therapy may be attempted. For salvage therapy, multiple drugs have been used simultaneously as a desperate measure with a success rate of about 40%.[112]Maertens J, Raad I, Petrikkos G, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004 Dec 1;39(11):1563-71.
https://academic.oup.com/cid/article/39/11/1563/462249
http://www.ncbi.nlm.nih.gov/pubmed/15578352?tool=bestpractice.com
[113]Kontoyiannis DP, Hachem R, Lewis RE, et al. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer. 2003 Jul 15;98(2):292-9.
http://onlinelibrary.wiley.com/doi/10.1002/cncr.11479/full
http://www.ncbi.nlm.nih.gov/pubmed/12872348?tool=bestpractice.com
Patients at high risk for invasive fungal infection with a suggestive CT scan and/or positive biomarkers (e.g., serum galactomannan) are candidates for preemptive therapy with voriconazole. Invasive procedures may not yield positive results or may be difficult to perform, so voriconazole is employed on the basis of a presumptive diagnosis.[114]Maertens J, Theunissen K, Verhoef G, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005 Nov 1;41(9):1242-50.
https://academic.oup.com/cid/article/41/9/1242/277461
http://www.ncbi.nlm.nih.gov/pubmed/16206097?tool=bestpractice.com
Surgical intervention may be indicated in patients with IA lesions contiguous with the great vessels or the pericardium or in severe hemoptysis from a single cavity or invasion of the chest wall. A single pulmonary lesion prior to intensive chemotherapy or stem cell transplantation is another relative indication for surgical resection.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[115]Caillot D, Mannone L, Cuisenier B, et al. Role of early diagnosis and aggressive surgery in the management of invasive pulmonary aspergillosis in neutropenic patients. Clin Microbiol Infect. 2001;7 Suppl 2:54-61.
http://www.ncbi.nlm.nih.gov/pubmed/11525219?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Treatment approach for invasive aspergillosisCreated by authors [Citation ends].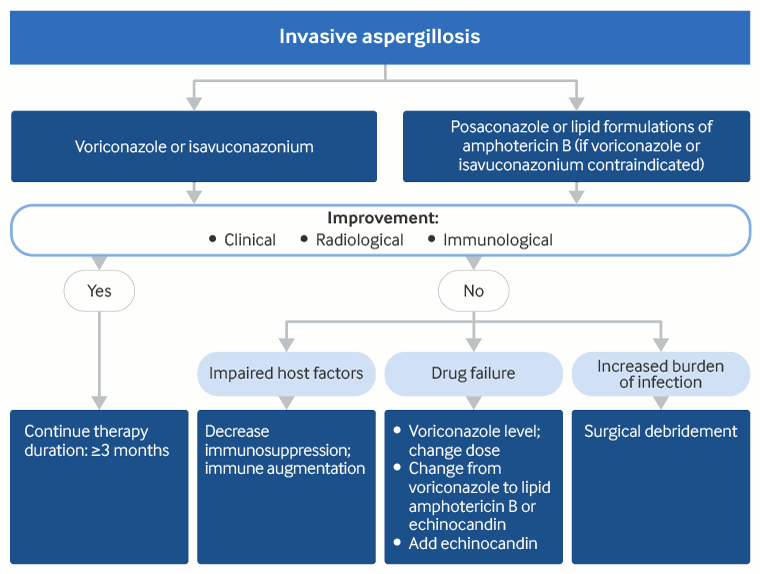
Chronic pulmonary aspergillosis
Aspergilloma:
Patients with stable, simple aspergilloma who have minimal or no symptoms require no treatment.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[116]Maghrabi F, Denning DW. The management of chronic pulmonary aspergillosis: the UK National Aspergillosis Centre approach. Curr Fungal Infect Rep. 2017;11(4):242-51.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5705730
http://www.ncbi.nlm.nih.gov/pubmed/29213345?tool=bestpractice.com
There is insufficient evidence that aspergilloma responds to antifungal agents. Therapy with intravenous amphotericin B has failed to show benefit in these patients. Penetration of amphotericin B within the lung cavities is suboptimal, and inhaled, intracavitary, and endobronchial instillation of amphotericin B have not shown consistent benefit.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
There are only anecdotal reports that treatment with intravenous itraconazole or voriconazole may be effective. In asymptomatic patients with aspergilloma, periodic monitoring with chest x-ray is appropriate.
Bronchial artery embolization may be helpful as a temporizing measure in symptomatic patients with severe hemoptysis, although the presence of massive collateral blood vessels makes the procedure suboptimal.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
[117]Uflacker R, Kaemmerer A, Picon PD, et al. Bronchial artery embolization in the management of hemoptysis: technical aspects and long-term results. Radiology. 1985 Dec;157(3):637-44.
http://www.ncbi.nlm.nih.gov/pubmed/4059552?tool=bestpractice.com
Surgical resection may be necessary in life-threatening hemoptysis; however, the postoperative morbidity/mortality remains a major concern. Complications include bleeding, bronchopulmonary fistula, empyema, and respiratory failure.[118]Chen JC, Chang YL, Luh SP, et al. Surgical treatment for pulmonary aspergilloma: a 28 year experience. Thorax. 1997 Sep;52(9):810-3.
http://www.ncbi.nlm.nih.gov/pubmed/9371213?tool=bestpractice.com
[119]Regnard JF, Icard P, Nicolosi M, et al. Aspergilloma: a series of 89 surgical cases. Ann Thorac Surg. 2000 Mar;69(3):898-903.
http://www.annalsthoracicsurgery.org/article/S0003-4975(99)01334-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/10750780?tool=bestpractice.com
Peri- and postoperative antifungal therapy is not routinely required, but guidelines suggest that if there is a moderate risk of surgical spillage of the aspergilloma, antifungal therapy with an azole or an echinocandin may be used to prevent Aspergillus empyema.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
Chronic cavitary pulmonary aspergillosis (CCPA):
Patients with CCPA are treated with antifungal therapy to halt progression, improve symptoms, and minimize hemoptysis.[116]Maghrabi F, Denning DW. The management of chronic pulmonary aspergillosis: the UK National Aspergillosis Centre approach. Curr Fungal Infect Rep. 2017;11(4):242-51.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5705730
http://www.ncbi.nlm.nih.gov/pubmed/29213345?tool=bestpractice.com
Oral itraconazole and oral voriconazole are the preferred options.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[116]Maghrabi F, Denning DW. The management of chronic pulmonary aspergillosis: the UK National Aspergillosis Centre approach. Curr Fungal Infect Rep. 2017;11(4):242-51.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5705730
http://www.ncbi.nlm.nih.gov/pubmed/29213345?tool=bestpractice.com
Oral posaconazole may also be considered as an alternative option if the preferred options cannot be used. Treatment is for a minimum of 6 months and may be extended in some patients. Treatment requires therapeutic drug monitoring and monitoring for drug-related side effects or toxicity.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
Patients should be managed by physicians with experience of antifungal therapy. Intravenous antifungal therapy may be considered in patients with progressive disease, or who are intolerant to azoles or develop resistance. An initial course of intravenous antifungal therapy may also be considered for some acutely ill patients.[2]Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4967602
http://www.ncbi.nlm.nih.gov/pubmed/27365388?tool=bestpractice.com
[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
Options include amphotericin B deoxycholate, liposomal amphotericin B, or a echinocandin (e.g., micafungin, caspofungin).
Chronic fibrosing pulmonary aspergillosis (CFPA):
CFPA generally results from untreated CCPA, though may represent treatment failure and disease progression. Antifungal treatment is the same as for CCPA, and may be continued indefinitely.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
[4]Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015 Mar;70(3):270-7.
https://thorax.bmj.com/content/70/3/270.long
http://www.ncbi.nlm.nih.gov/pubmed/25354514?tool=bestpractice.com
Subacute invasive aspergillosis (SAIA):
SAIA should be treated in the same way as acute IA above.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
Aspergillus nodule:
Aspergillus nodules are diagnosed after excision biopsy, usually following suspicion for malignancy. Single nodules that are completely excised may not need antifungal therapy, unless the patient is immunocompromised.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
[4]Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015 Mar;70(3):270-7.
https://thorax.bmj.com/content/70/3/270.long
http://www.ncbi.nlm.nih.gov/pubmed/25354514?tool=bestpractice.com
Single nodules that are not completely resected should be closely monitored.
Antifungal therapy may also be considered in patients with multiple nodules.[3]Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68.
https://erj.ersjournals.com/content/47/1/45.long
http://www.ncbi.nlm.nih.gov/pubmed/26699723?tool=bestpractice.com
