Criteria
Consensus definitions of invasive fungal disease from the European Organisation for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium[95]
The European Organisation for Research and Treatment of Cancer and the Mycoses Study Group provide consensus definitions for 'proven', 'probable', and 'possible' invasive fungal diseases (including invasive aspergillosis). The 'probable' and 'possible' categories apply only to immunocompromised patients, whereas the 'proven' category can apply to any patient, regardless of whether they are immunocompromised.
Criteria for proven invasive fungal disease:
Histopathological, cytopathological, or direct microscopic examination of a specimen obtained by needle aspiration or biopsy in which hyphae are seen accompanied by evidence of tissue damage
Culture of a specimen obtained by a sterile procedure from a normally sterile and clinically or radiologically abnormal site consistent with an infectious disease process, excluding bronchoalveolar lavage fluid (BAL), a paranasal or mastoid sinus cavity specimen, and urine
Blood culture that yields a mould (e.g., Fusarium species) in the context of a compatible infectious disease process
Amplification of fungal DNA by polymerase chain reaction (PCR) combined with DNA sequencing when moulds are seen in formalin-fixed paraffin-embedded tissue
Probable invasive pulmonary mould disease:
Requires the presence of one host factor, one clinical feature, and mycological evidence (see below).
Possible invasive pulmonary mould disease:
Presence of one host factor and one clinical feature, but mycological evidence has not been found.
Host factors
Recent history of neutropenia (<0.5 × 10⁹ neutrophils/L [<500 neutrophils/mm³] for >10 days) temporally related to the onset of fungal disease
Haematological malignancy
Receipt of an allogeneic stem cell transplant
Receipt of a solid organ transplant
Prolonged use of corticosteroids (excluding among patients with allergic bronchopulmonary aspergillosis) at a therapeutic dose of ≥0.3 mg/kg/day for ≥3 weeks in the past 60 days
Treatment with other recognised T-cell immunosuppressants, such as calcineurin inhibitors, tumour necrosis factor-alpha blockers, lymphocyte-specific monoclonal antibodies, immunosuppressive nucleoside analogues during the past 90 days
Treatment with recognised B-cell immunosuppressants, such as Bruton's tyrosine kinase inhibitors, e.g., ibrutinib
Inherited severe immunodeficiency (such as chronic granulomatous disease or severe combined immunodeficiency)
Acute graft-versus-host disease grade III or IV involving the gut, lungs, or liver that is refractory to first-line treatment with steroids.
Clinical features
Pulmonary aspergillosis (presence of one of the following four patterns on CT):
Dense, well circumscribed lesion(s) with or without a halo sign [Figure caption and citation for the preceding image starts]: 'Halo' sign in early pulmonary aspergillosisFrom the collection of Dr P. Chandrasekar; used with permission [Citation ends].

Air-crescent sign [Figure caption and citation for the preceding image starts]: 'Air-crescent' sign in late pulmonary aspergillosisFrom the collection of Dr P. Chandrasekar; used with permission [Citation ends].
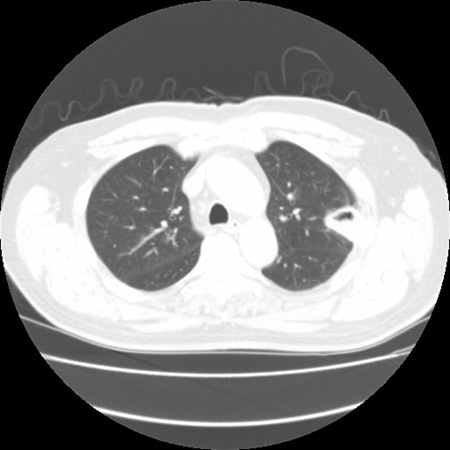
Cavity
Wedge-shaped and segmental or lobar consolidation
Tracheobronchitis:
Tracheobronchial ulceration, nodule, pseudomembrane, plaque, or eschar seen on bronchoscopic analysis
Sino-nasal infection:
Acute localised pain (including pain radiating to the eye)
Nasal ulcer with black eschar
Extension from the paranasal sinus across bony barriers, including into the orbit
Central nervous system infection (one of the following two signs):
Focal lesions on imaging
Meningeal enhancement on MRI or CT.
Mycological evidence
Aspergillus recovered by culture from sputum, BAL fluid, bronchial brush, or aspirate
Microscopic detection of fungal elements indicating a mould from sputum, BAL fluid, bronchial brush, or aspirate
Aspergillus galactomannan antigen detected in plasma, serum, BAL fluid, or cerebrospinal fluid
Two or more consecutive PCR tests positive from plasma, serum, or whole blood, or two or more duplicate PCR tests from BAL fluid positive, or at least one PCR test positive in plasma, serum, or whole blood and one PCR test positive in BAL fluid.
Diagnostic criteria for chronic pulmonary aspergillosis from the European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society[3]
Simple aspergilloma
Single pulmonary cavity containing a fungal ball, with serological or microbiological evidence implicating Aspergillus species in a non-immunocompromised patient with minor or no symptoms and no radiological progression over at least 3 months of observation.
Chronic cavitary pulmonary aspergillosis (CCPA)
One or more pulmonary cavities (with either a thin or thick wall) possibly containing one or more aspergillomas or irregular intraluminal material, with serological or microbiological evidence implicating Aspergillus species with significant pulmonary and/or systemic symptoms and overt radiological progression (new cavities, increasing pericavitary infiltrates, or increasing fibrosis) over at least 3 months of observation.
Chronic fibrosing pulmonary aspergillosis (CFPA)
Severe fibrotic destruction of at least two lobes of lung complicating CCPA leading to a major loss of lung function. Severe fibrotic destruction of one lobe with a cavity is simply referred to as CCPA affecting that lobe. Usually the fibrosis is manifest as consolidation, but large cavities with surrounding fibrosis may be seen.
Aspergillus nodule
One or more nodules which may or may not cavitate are an unusual form of CPA. They may mimic tuberculoma, carcinoma of the lung, coccidioidomycosis, and other diagnoses and can only be definitively diagnosed on histology. Tissue invasion is not demonstrated, although necrosis is frequent.
Subacute invasive aspergillosis
Invasive aspergillosis, usually in mildly immunocompromised patients, occurring over 1-3 months, with variable radiological features including cavitation, nodules, progressive consolidation with 'abscess formation'. Biopsy shows hyphae in invading lung tissue and microbiological investigations reflect those in invasive aspergillosis, notably positive Aspergillus galactomannan antigen in blood (or respiratory fluids).
Use of this content is subject to our disclaimer