The main elements in the management of new-onset atrial fibrillation (AF) are:[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
[102]Kanji S, Stewart R, Fergusson DA, et al. Treatment of new-onset atrial fibrillation in noncardiac intensive care unit patients: a systematic review of randomized controlled trials. Crit Care Med. 2008 May;36(5):1620-4.
http://www.ncbi.nlm.nih.gov/pubmed/18434899?tool=bestpractice.com
[103]Okcun B, Yigit Z, Yildiz A, et al. What should be the primary treatment in atrial fibrillation: ventricular rate control or sinus rhythm control with long-term anticoagulation? J Int Med Res. 2009 Mar-Apr;37(2):464-71.
http://www.ncbi.nlm.nih.gov/pubmed/19383241?tool=bestpractice.com
[104]Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51.
https://www.nejm.org/doi/10.1056/NEJMoa0905561
http://www.ncbi.nlm.nih.gov/pubmed/19717844?tool=bestpractice.com
Ventricular rate control
Restoration and maintenance of sinus rhythm
Prevention of thromboembolic events.
As an initial step, identification and treatment of any potential triggers of new-onset AF is very important, because rate and rhythm control measures are less likely to succeed if the acute precipitant persists.[43]Chyou JY, Barkoudah E, Dukes JW, et al. Atrial fibrillation occurring during acute hospitalization: a scientific statement from the American Heart Association. Circulation. 2023 Apr 11;147(15):e676-98.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001133
http://www.ncbi.nlm.nih.gov/pubmed/36912134?tool=bestpractice.com
Ventricular rate control
Management of new-onset AF depends on the nature of its presentation, so the urgency of the treatment required should be assessed. Most cases of new-onset AF revert to sinus rhythm spontaneously, but treatment may still be needed to restore adequate ventricular rate, with drugs such as beta-blockers, calcium-channel blockers, and occasionally digoxin.[105]Prystowsky EN, Benson DW Jr, Fuster V, et al. Management of patients with atrial fibrillation: a statement for healthcare professionals from the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation. 1996 Mar 15;93(6):1262-77.
https://www.ahajournals.org/doi/10.1161/01.cir.93.6.1262
http://www.ncbi.nlm.nih.gov/pubmed/8653857?tool=bestpractice.com
Digoxin is not considered a first-line agent for the purpose of rate control, but it can be useful in patients with heart failure. One study explored whether digoxin use was independently associated with increased mortality in patients with AF; compared with propensity score–matched control participants, the risk of death and sudden death was significantly higher in new digoxin users.[106]Lopes RD, Rordorf R, De Ferrari GM, et al; ARISTOTLE Committees and Investigators. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018 Mar 13;71(10):1063-74.
https://www.sciencedirect.com/science/article/pii/S0735109718301037?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/29519345?tool=bestpractice.com
In patients with AF taking digoxin, the risk of death was independently related to serum digoxin concentration and was highest in patients with concentrations of ≥1.2 ng/mL (≥1.54 nmol/L).[106]Lopes RD, Rordorf R, De Ferrari GM, et al; ARISTOTLE Committees and Investigators. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018 Mar 13;71(10):1063-74.
https://www.sciencedirect.com/science/article/pii/S0735109718301037?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/29519345?tool=bestpractice.com
Cases that revert spontaneously usually do so in the first 24 hours.[105]Prystowsky EN, Benson DW Jr, Fuster V, et al. Management of patients with atrial fibrillation: a statement for healthcare professionals from the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation. 1996 Mar 15;93(6):1262-77.
https://www.ahajournals.org/doi/10.1161/01.cir.93.6.1262
http://www.ncbi.nlm.nih.gov/pubmed/8653857?tool=bestpractice.com
Patients whose AF does not revert, or who present with significant symptoms and hemodynamic instability, may require either direct current (DC) cardioversion or pharmacologic cardioversion.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
[105]Prystowsky EN, Benson DW Jr, Fuster V, et al. Management of patients with atrial fibrillation: a statement for healthcare professionals from the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation. 1996 Mar 15;93(6):1262-77.
https://www.ahajournals.org/doi/10.1161/01.cir.93.6.1262
http://www.ncbi.nlm.nih.gov/pubmed/8653857?tool=bestpractice.com
There is no significant difference in terms of outcome between DC and pharmacologic cardioversion. Timing of cardioversion in patients who are hemodynamically stable and minimally symptomatic is a topic of research. Some studies suggest no benefit of early cardioversion over a "wait and see" approach; others suggest outcomes are improved when cardioversion is performed early.[107]Pope MK, Hall TS, Schirripa V, et al. Cardioversion in patients with newly diagnosed non-valvular atrial fibrillation: observational study using prospectively collected registry data. BMJ. 2021 Oct 27;375:e066450.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8548918
http://www.ncbi.nlm.nih.gov/pubmed/34706884?tool=bestpractice.com
[108]Pluymaekers NAHA, Dudink EAMP, Luermans JGLM, et al. Early or delayed cardioversion in recent-onset atrial fibrillation. N Engl J Med. 2019 Apr 18;380(16):1499-1508.
https://www.nejm.org/doi/full/10.1056/NEJMc1906729
http://www.ncbi.nlm.nih.gov/pubmed/30883054?tool=bestpractice.com
New-onset AF may be the first symptomatic presentation of paroxysmal AF, in patients with high risk substrates of stroke, it is reasonable to perform transesophageal echocardiography (TEE) before cardioversion to rule out left atrial (LA) clots.[109]Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001 May 10;344(19):1411-20.
https://www.nejm.org/doi/10.1056/NEJM200105103441901
http://www.ncbi.nlm.nih.gov/pubmed/11346805?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Transesophageal echocardiogram showing left atrial appendage clot. LA=left atrium; LAA=left atrial appendage; LV=left ventricleFrom the collection of Dr Bharat Kantharia [Citation ends].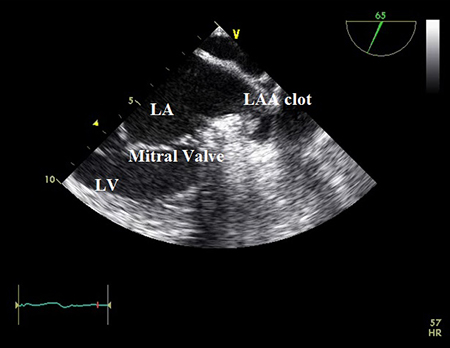
Restoration and maintenance of sinus rhythm
Depending on further risks for AF, patients may require treatment with an antiarrhythmic agent to prevent AF and maintain sinus rhythm once it has been restored.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
Antiarrhythmics are reasonable for long-term maintenance of sinus rhythm for patients with AF who are not candidates for, or decline, catheter ablation or who prefer antiarrhythmic therapy.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
For more information on this see Established atrial fibrillation.
One multicenter randomized control trial looked at outcomes of a strategy of initiating rhythm-control therapy in patients with early AF (of less than 1 year duration). This study identified a reduced risk of death, from cardiovascular causes, stroke, and hospitalization for heart failure or acute coronary syndrome over 5 years in patients who received early rhythm-control therapy.[110]Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020 Oct 1;383(14):1305-16.
https://www.nejm.org/doi/full/10.1056/NEJMoa2019422
http://www.ncbi.nlm.nih.gov/pubmed/32865375?tool=bestpractice.com
Prevention of thromboembolic events
Anticoagulation is recommended in patients with AF irrespective of whether the AF is paroxysmal, persistent, or permanent, due to the high risk of thromboembolic events. Many patients require anticoagulation before, during, and after cardioversion to prevent thromboembolism.[111]Alpert JS. Take-home messages from the recently updated AHA/ACC guidelines for atrial fibrillation. Am J Med. 2019 Dec;132(12):1363-4.
http://www.ncbi.nlm.nih.gov/pubmed/31401166?tool=bestpractice.com
[112]Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016 Feb 16;37(20):1591-602.
www.doi.org/10.1093/eurheartj/ehw007
http://www.ncbi.nlm.nih.gov/pubmed/26888184?tool=bestpractice.com
[113]Lilli A, Di Cori A, Zacà V. Thromboembolic risk and effect of oral anticoagulation according to atrial fibrillation patterns: a systematic review and meta-analysis. Clin Cardiol. 2017 Sep;40(9):641-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6490401
http://www.ncbi.nlm.nih.gov/pubmed/28471498?tool=bestpractice.com
Selection of stroke risk reduction therapy should be guided by the patient’s risk of stroke, risks of bleeding with therapy, and their individual preferences.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
The key options for anticoagulation are a vitamin K antagonist such as warfarin, or a direct oral anticoagulant (DOAC) such as dabigatran, rivaroxaban, apixaban, or edoxaban. Both vitamin K antagonists and DOACs are approved as efficacious agents for stroke prevention in AF and in patients undergoing cardioversion.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
[11]Katsanos AH, Kamel H, Healey JS, et al. Stroke prevention in atrial fibrillation: looking forward. Circulation. 2020 Dec 15;142(24):2371-88.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.049768
http://www.ncbi.nlm.nih.gov/pubmed/33315494?tool=bestpractice.com
In patients with AF who are candidates for anticoagulation and do not have either moderate-severe rheumatic mitral stenosis or mechanical heart valves, DOACs are recommended over warfarin to reduce the risk of mortality, stroke, systemic embolism, and intracranial hemorrhage.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
DOACs are nonvitamin K-dependent and they fall into two classes: oral direct thrombin inhibitors (e.g., dabigatran) and oral direct factor Xa inhibitors (e.g., rivaroxaban, apixaban, edoxaban).[114]O'Dell KM, Igawa D, Hsin J. New oral anticoagulants for atrial fibrillation: a review of clinical trials. Clin Ther. 2012 Apr;34(4):894-901.
http://www.ncbi.nlm.nih.gov/pubmed/22417716?tool=bestpractice.com
DOACs are approved for stroke prevention and have a more favorable side-effect profile than vitamin K antagonists in patients with nonvalvular AF. The fast onset of action of DOACs is also a benefit, which could reduce delays to cardioversion, as well as negating the need for an interim alternative anticoagulant.[115]Gibson CM, Basto AN, Howard ML. Direct oral anticoagulants in cardioversion: a review of current evidence. Ann Pharmacother. 2018 Mar;52(3):277-84.
http://www.ncbi.nlm.nih.gov/pubmed/29025267?tool=bestpractice.com
DOACs are recommended over warfarin in eligible patients (i.e., patients who do not have moderate-to-severe mitral stenosis or a mechanical heart valve).[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
[116]National Institute for Health and Care Excellence. Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation. Jul 2021 [internet publication].
https://www.nice.org.uk/guidance/TA256
[117]Mincu RI, Mahabadi AA, Totzeck M, et al. Novel anticoagulants versus vitamin K antagonists for cardioversion of non- valvular atrial fibrillation - a meta-analysis of more than 17000 patients. Sci Rep. 2019 Feb 28;9(1):3011.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6395612
http://www.ncbi.nlm.nih.gov/pubmed/30816247?tool=bestpractice.com
[118]Telles-Garcia N, Dahal K, Kocherla C, et al. Non-vitamin K antagonists oral anticoagulants are as safe and effective as warfarin for cardioversion of atrial fibrillation: A systematic review and meta-analysis. Int J Cardiol. 2018 Oct 1;268:143-8.
http://www.ncbi.nlm.nih.gov/pubmed/30041779?tool=bestpractice.com
The use of DOACs in this setting is supported by data from several studies, most notably:
The GARFIELD-AF study: a prospective registry of more than 52,000 patients with newly diagnosed AF. Data from GARFIELD-AF suggest that DOACs are associated with lower risks of all-cause death and bleeding than vitamin K antagonists such as warfarin.[119]Bassand JP, Virdone S, Badoz M, et al. Bleeding and related mortality with NOACs and VKAs in newly diagnosed atrial fibrillation: results from the GARFIELD-AF registry. Blood Adv. 2021 Feb 23;5(4):1081-91.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7903226
http://www.ncbi.nlm.nih.gov/pubmed/33606006?tool=bestpractice.com
The RE-LY trial: compared dabigatran, an oral direct thrombin inhibitor, with warfarin. The trial included 18,113 patients and had a median follow-up of 2 years.[104]Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51.
https://www.nejm.org/doi/10.1056/NEJMoa0905561
http://www.ncbi.nlm.nih.gov/pubmed/19717844?tool=bestpractice.com
The results of RE-LY suggest that compared with warfarin, dabigatran showed noninferiority at a low dose, and superiority at a high dose regarding rates of stroke and systemic embolism (warfarin 1.69% per year, lower-dose dabigatran 1.53% per year, and higher-dose dabigatran 1.11% per year for primary end point of stroke and systemic embolism). Adverse bleeding event rates were lower with a lower dose and similar with a higher dose of dabigatran compared with warfarin. The rate of myocardial infarction was higher with both doses of dabigatran than with warfarin.[104]Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51.
https://www.nejm.org/doi/10.1056/NEJMoa0905561
http://www.ncbi.nlm.nih.gov/pubmed/19717844?tool=bestpractice.com
[114]O'Dell KM, Igawa D, Hsin J. New oral anticoagulants for atrial fibrillation: a review of clinical trials. Clin Ther. 2012 Apr;34(4):894-901.
http://www.ncbi.nlm.nih.gov/pubmed/22417716?tool=bestpractice.com
[120]Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012 Aug 1;110(3):453-60.
http://www.ncbi.nlm.nih.gov/pubmed/22537354?tool=bestpractice.com
The ROCKET AF, ARISTOTLE, and ENGAGE-AF trials: the oral direct factor Xa inhibitors rivaroxaban, apixaban, and edoxaban were compared with warfarin for stroke prevention in patients with nonvalvular AF in the ROCKET AF (14,264 patients, median follow-up of 1.9 years), ARISTOTLE (18,201 patients, median follow-up of 1.8 years), and the ENGAGE-AF (21,105 patients, median follow-up of 2.8 years) trials, respectively.[121]Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
http://www.nejm.org/doi/full/10.1056/NEJMoa1009638#t=article
http://www.ncbi.nlm.nih.gov/pubmed/21830957?tool=bestpractice.com
[122]Halperin JL, Hankey GJ, Wojdyla DM, et al; ROCKET AF Steering Committee and Investigators. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation. 2014 Jul 8;130(2):138-46.
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.113.005008?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
http://www.ncbi.nlm.nih.gov/pubmed/24895454?tool=bestpractice.com
[123]Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
http://www.nejm.org/doi/full/10.1056/NEJMoa1107039#t=article
http://www.ncbi.nlm.nih.gov/pubmed/21870978?tool=bestpractice.com
[124]Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
http://www.nejm.org/doi/full/10.1056/NEJMoa1310907#t=article
http://www.ncbi.nlm.nih.gov/pubmed/24251359?tool=bestpractice.com
The primary end point of stroke and/or systemic embolism were 1.7% per year with rivaroxaban compared with 2.2% per year with warfarin in the ROCKET AF trial, 1.3% per year with apixaban compared with 1.6% per year with warfarin in the ARISTOTLE trial, and 1.6% per year with a lower dose and 1.2% per year with a higher dose of edoxaban compared with 1.5% per year with warfarin in the ENGAGE-AF trial.[121]Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
http://www.nejm.org/doi/full/10.1056/NEJMoa1009638#t=article
http://www.ncbi.nlm.nih.gov/pubmed/21830957?tool=bestpractice.com
[122]Halperin JL, Hankey GJ, Wojdyla DM, et al; ROCKET AF Steering Committee and Investigators. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation. 2014 Jul 8;130(2):138-46.
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.113.005008?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
http://www.ncbi.nlm.nih.gov/pubmed/24895454?tool=bestpractice.com
[123]Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
http://www.nejm.org/doi/full/10.1056/NEJMoa1107039#t=article
http://www.ncbi.nlm.nih.gov/pubmed/21870978?tool=bestpractice.com
[124]Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
http://www.nejm.org/doi/full/10.1056/NEJMoa1310907#t=article
http://www.ncbi.nlm.nih.gov/pubmed/24251359?tool=bestpractice.com
One prospective cohort study comparing rivaroxaban to vitamin K antagonists for bleeding risk in patients over 80 years of age, with nonvalvular AF: the primary end point of major bleeding occurred in 6.5% of patients treated with rivaroxaban and 11.2% treated with vitamin K antagonists.[125]Hanon O, Vidal JS, Pisica-Donose G, et al. Bleeding risk with rivaroxaban compared with vitamin K antagonists in patients aged 80 years or older with atrial fibrillation. Heart. 2021 Sep;107(17):1376-82.
http://www.ncbi.nlm.nih.gov/pubmed/33262185?tool=bestpractice.com
Fatal bleeding occurred in 0.9% of rivaroxaban patients and 3.3% of patients treated with vitamin K antagonists.[125]Hanon O, Vidal JS, Pisica-Donose G, et al. Bleeding risk with rivaroxaban compared with vitamin K antagonists in patients aged 80 years or older with atrial fibrillation. Heart. 2021 Sep;107(17):1376-82.
http://www.ncbi.nlm.nih.gov/pubmed/33262185?tool=bestpractice.com
These studies, together with results from meta-analyses and systematic reviews, have shown that DOACs are noninferior to warfarin for stroke prevention in patients with nonvalvular AF, and may be associated with a reduced risk of fatal bleeding.[120]Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012 Aug 1;110(3):453-60.
http://www.ncbi.nlm.nih.gov/pubmed/22537354?tool=bestpractice.com
[125]Hanon O, Vidal JS, Pisica-Donose G, et al. Bleeding risk with rivaroxaban compared with vitamin K antagonists in patients aged 80 years or older with atrial fibrillation. Heart. 2021 Sep;107(17):1376-82.
http://www.ncbi.nlm.nih.gov/pubmed/33262185?tool=bestpractice.com
[126]Caldeira D, Rodrigues FB, Barra M, et al. Non-vitamin K antagonist oral anticoagulants and major bleeding-related fatality in patients with atrial fibrillation and venous thromboembolism: a systematic review and meta-analysis. Heart. 2015;101:1204-1211.
http://www.ncbi.nlm.nih.gov/pubmed/26037103?tool=bestpractice.com
[127]Lowenstern A, Al-Khatib SM, Sharan L, et al. Interventions for preventing thromboembolic events in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2018 Dec 4;169(11):774-87.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6825839
http://www.ncbi.nlm.nih.gov/pubmed/30383133?tool=bestpractice.com
[128]Ng SS, Lai NM, Nathisuwan S, et al. Comparative efficacy and safety of warfarin care bundles and novel oral anticoagulants in patients with atrial fibrillation: a systematic review and network meta-analysis. Sci Rep. 2020 Jan 20;10(1):662.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6971267
http://www.ncbi.nlm.nih.gov/pubmed/31959803?tool=bestpractice.com
[  ]
How do factor Xa inhibitors compare with warfarin for prevention of cerebral and systemic embolism in people with atrial fibrillation (AF)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2101/fullShow me the answer[Evidence A]cdfbf4fa-74e0-4e6b-bec7-0b74d8684beaccaAHow do factor Xa inhibitors compare with warfarin for prevention of cerebral and systemic embolism in people with atrial fibrillation (AF)? It is, therefore, reasonable to use DOACs as first-line agents or as a subsequent replacement for warfarin in patients with AF.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
Based on the current evidence, dabigatran is favored as a first-line agent or subsequent replacement for warfarin in suitable patients who do not have marked renal insufficiency, and who do not have mechanical prosthetic valves.[114]O'Dell KM, Igawa D, Hsin J. New oral anticoagulants for atrial fibrillation: a review of clinical trials. Clin Ther. 2012 Apr;34(4):894-901.
http://www.ncbi.nlm.nih.gov/pubmed/22417716?tool=bestpractice.com
[120]Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012 Aug 1;110(3):453-60.
http://www.ncbi.nlm.nih.gov/pubmed/22537354?tool=bestpractice.com
]
How do factor Xa inhibitors compare with warfarin for prevention of cerebral and systemic embolism in people with atrial fibrillation (AF)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2101/fullShow me the answer[Evidence A]cdfbf4fa-74e0-4e6b-bec7-0b74d8684beaccaAHow do factor Xa inhibitors compare with warfarin for prevention of cerebral and systemic embolism in people with atrial fibrillation (AF)? It is, therefore, reasonable to use DOACs as first-line agents or as a subsequent replacement for warfarin in patients with AF.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
Based on the current evidence, dabigatran is favored as a first-line agent or subsequent replacement for warfarin in suitable patients who do not have marked renal insufficiency, and who do not have mechanical prosthetic valves.[114]O'Dell KM, Igawa D, Hsin J. New oral anticoagulants for atrial fibrillation: a review of clinical trials. Clin Ther. 2012 Apr;34(4):894-901.
http://www.ncbi.nlm.nih.gov/pubmed/22417716?tool=bestpractice.com
[120]Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012 Aug 1;110(3):453-60.
http://www.ncbi.nlm.nih.gov/pubmed/22537354?tool=bestpractice.com
DOACs are generally safe in older patients; however, dabigatran may be associated with an increased risk of gastrointestinal bleeding compared with warfarin in patients ages >75 years.[129]Sharma M, Cornelius VR, Patel JP, et al. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: systematic review and meta-analysis. Circulation. 2015 Jul 21;132(3):194-204.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4765082
http://www.ncbi.nlm.nih.gov/pubmed/25995317?tool=bestpractice.com
[130]Gommans E, Grouls RJE, Kerkhof D, et al. Dabigatran trough concentrations in very elderly patients. Eur J Hosp Pharm. 2021 Jul;28(4):231-3.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8239269
http://www.ncbi.nlm.nih.gov/pubmed/32978221?tool=bestpractice.com
DOACs should be used with caution in patients with renal impairment. In patients with nonvalvular AF and mild or moderate renal impairment, the use of DOACs has been found to be associated with a reduced risk of stroke or systemic embolism and a reduced risk of major bleeding compared with warfarin, which suggests a favorable risk profile of these agents in patients with mild-to-moderate renal disease.[131]Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016 Jan 1;117(1):69-75.
http://www.ncbi.nlm.nih.gov/pubmed/26698882?tool=bestpractice.com
Some DOACs may be used in patients with renal impairment. If a DOAC is suitable, depending on the degree of renal impairment and the indication for use, a dose adjustment may be required; consult the prescribing information for specific guidance on use in patients with renal impairment. DOACs should not be used in patients with mechanical prosthetic valves or moderate-to-severe mitral stenosis, due to an increased risk of stroke, heart attack, and blood clot in these patients, nor should they be used in combination with heparin (including low molecular weight heparin), heparin derivatives, or warfarin.[111]Alpert JS. Take-home messages from the recently updated AHA/ACC guidelines for atrial fibrillation. Am J Med. 2019 Dec;132(12):1363-4.
http://www.ncbi.nlm.nih.gov/pubmed/31401166?tool=bestpractice.com
Patients with diabetes are at risk of complications of diabetes when treated with oral anticoagulants; this risk seems to be lower with DOACs compared with vitamin K antagonists. Dabigatran is favored for its efficacy and lower rates of adverse effects in this patient group.[132]Jin H, Zhu K, Wang L, et al. A network meta-analysis of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and diabetes mellitus. Acta Cardiol. 2021 Nov;76(9):960-9.
http://www.ncbi.nlm.nih.gov/pubmed/33432890?tool=bestpractice.com
[133]Huang HK, Liu PP, Lin SM, et al. Diabetes-related complications and mortality in patients with atrial fibrillation receiving different oral anticoagulants: a nationwide analysis. Ann Intern Med. 2022 Apr;175(4):490-8.
http://www.ncbi.nlm.nih.gov/pubmed/35157495?tool=bestpractice.com
Warfarin remains the first-line therapy in patients with AF and moderate-severe rheumatic mitral stenosis or mechanical heart valves. Warfarin takes several days to have a therapeutic effect, so patients presenting with new-onset AF treated with warfarin are treated with intravenous heparin (activated partial thromboplastin time [aPTT] of 45-60 seconds) or subcutaneous low-molecular-weight heparin while they are awaiting cardioversion and being evaluated for long-term anticoagulation. Once the patient is established on warfarin, the efficacy and safety of anticoagulation with warfarin is highly dependent on the quality of anticoagulation control, as reflected by the average time in therapeutic range (TTR) of INR 2-3. The SAMe-TT₂R₂ scoring system (based on sex, age, medical history, treatment interactions, tobacco use, and race) is a tool that may help identify anticoagulation-naive patients who are less likely to maintain TTR >70% and who should, therefore, be managed with DOACs instead of warfarin.[134]Gallego P, Roldán V, Marin F, et al. SAMe-TT2R2 score, time in therapeutic range, and outcomes in anticoagulated patients with atrial fibrillation. Am J Med. 2014 Nov;127(11):1083-8.
https://www.amjmed.com/article/S0002-9343(14)00459-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/24858062?tool=bestpractice.com
[135]Lip GY, Haguenoer K, Saint-Etienne C, et al. Relationship of the SAMe-TT(2)R(2) score to poor-quality anticoagulation, stroke, clinically relevant bleeding, and mortality in patients with atrial fibrillation. Chest. 2014 Sep;146(3):719-26.
http://www.ncbi.nlm.nih.gov/pubmed/24722973?tool=bestpractice.com
SAMe-TT₂R₂ score
Opens in new window
If there are no risk factors for stroke, aspirin (either alone or in combination with clopidogrel) is not recommended to reduce the risk of stroke or to prevent thromboembolic events.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
The choice of anticoagulation strategy depends on the presentation. Factors in the patient's presentation and diagnostic assessment that guide appropriate treatment include the following:
Whether the patient is hemodynamically stable or unstable
If hemodynamically stable, whether the patient is symptomatic or asymptomatic
If symptomatic, the onset of the symptoms (<48 hours, ≥48 hours, or unknown)
The presence of associated heart failure
The presence of a thrombus on TEE
If a thrombus is absent on TEE, thromboembolic risk is stratified.
[
Atrial Fibrillation CHA(2)DS(2)-VASc Score for Stroke Risk
Opens in new window
]
If the AF is of unknown duration, or if TEE cannot be performed, the patient should be treated as for presumed thrombus and recommendations for confirmed LA thrombus should be followed.
In the setting of AF, the left atrial appendage can play a role in blood stasis and clot formation, and consequently be a source of emboli.[11]Katsanos AH, Kamel H, Healey JS, et al. Stroke prevention in atrial fibrillation: looking forward. Circulation. 2020 Dec 15;142(24):2371-88.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.049768
http://www.ncbi.nlm.nih.gov/pubmed/33315494?tool=bestpractice.com
Although oral anticoagulation is the standard of care to reduce the risk of ischemic stroke in patients with AF, it is contraindicated in some patients due to an excess risk of major bleeding.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
Left atrial appendage occlusion (LAAO) may be considered as an alternative for stroke prevention in patients with a moderate to high risk of stroke (CHA₂DS₂-VASc score ≥2) when there are absolute contraindications to the use of anticoagulants (due to a nonreversible cause), or the risk of bleeding outweighs the benefits.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
[2]Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021 Feb 1;42(5):373-498.
https://academic.oup.com/eurheartj/article/42/5/373/5899003?login=false
[18]National Institute for Health and Care Excellence. Atrial fibrillation: diagnosis and management. Jun 2021 [internet publication].
https://www.nice.org.uk/guidance/ng196
[136]Glikson M, Wolff R, Hindricks G, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion - an update. Europace. 2020 Feb 1;22(2):184.
https://academic.oup.com/europace/article/22/2/184/5557705
For more information on this treatment see Established atrial fibrillation.
Hemodynamically stable AF: symptomatic
Patients require rate-control therapy until cardioversion is successful. If there is no evidence of heart failure, beta-blockers (e.g., intravenous esmolol, propranolol, metoprolol; oral atenolol, metoprolol, nadolol, propranolol, bisoprolol, carvedilol) or nondihydropyridine calcium-channel blockers (i.e., diltiazem, verapamil) are the preferred choice.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
Nondihydropyridine calcium-channel blockers are useful in ventricular rate control in the absence of preexcitation. They provide reasonable rate control and also improve AF-related symptoms compared with beta-blockers.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
If rate control is inadequate with monotherapy, a combination of a beta-blocker and calcium-channel blockers may be used. Patients should be carefully monitored to prevent excess atrioventricular nodal blockade.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
If there is evidence of decompensated heart failure, nondihydropyridine calcium-channel blockers are contraindicated.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
Certain beta-blockers, digoxin, or amiodarone may be used for rate control in patients with AF and heart failure.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
[15]Gorenek B, Halvorsen S, Kudaiberdieva G, et al. Atrial fibrillation in acute heart failure: A position statement from the Acute Cardiovascular Care Association and European Heart Rhythm Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020 Jun;9(4):348-57.
https://academic.oup.com/ehjacc/article/9/4/348/5950241
http://www.ncbi.nlm.nih.gov/pubmed/31976747?tool=bestpractice.com
Patients presenting with new-onset AF of <48 hours' duration and no evidence of LA thrombus on TEE should have DC or pharmacologic cardioversion. DC cardioversion is fast, safe, and efficient. Pharmacologic cardioversion is accomplished with the use of antiarrhythmic agents.[137]Heldal M, Atar D. Pharmacological conversion of recent-onset atrial fibrillation: a systematic review. Scand Cardiovasc J Suppl. 2013 Feb;47(1):2-10.
http://www.ncbi.nlm.nih.gov/pubmed/23067130?tool=bestpractice.com
However, these must be used with caution, as they may cause bradycardia or tachyarrhythmias. Antiarrhythmic agents with variable, but demonstrated, efficacy for cardioversion of new-onset AF include flecainide, propafenone, ibutilide, and amiodarone.[138]Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007 Sep 6;357(10):987-99.
http://www.nejm.org/doi/full/10.1056/NEJMoa054686#t=article
http://www.ncbi.nlm.nih.gov/pubmed/17804843?tool=bestpractice.com
[139]Le Heuzey JY, De Ferrari GM, Radzik D, et al. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010 Jun 1;21(6):597-605.
http://www.ncbi.nlm.nih.gov/pubmed/20384650?tool=bestpractice.com
[140]Hohnloser SH, Crijns HJ, van Eickels M, et al; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009 Feb 12;360(7):668-78.
https://www.nejm.org/doi/10.1056/NEJMoa0803778?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov
http://www.ncbi.nlm.nih.gov/pubmed/19213680?tool=bestpractice.com
Class III agents (including amiodarone and ibutilide) are less efficacious than class IC agents (flecainide and propafenone) in conversion to sinus rhythm.[141]Kochiadakis GE, Igoumenidis NE, Hamilos ME, et al. A comparative study of the efficacy and safety of procainamide versus propafenone versus amiodarone for the conversion of recent-onset atrial fibrillation. Am J Cardiol. 2007 Jun 15;99(12):1721-5.
https://www.ajconline.org/article/S0002-9149(07)00508-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/17560882?tool=bestpractice.com
[142]Xanthos T, Bassiakou E, Vlachos IS, et al. Intravenous and oral administration of amiodarone for the treatment of recent onset atrial fibrillation after digoxin administration. Int J Cardiol. 2007 Oct 18;121(3):291-5.
http://www.ncbi.nlm.nih.gov/pubmed/17434635?tool=bestpractice.com
[143]Xanthos T, Prapa V, Papadimitriou D, et al. Comparative study of intravenous amiodarone and procainamide in the treatment of atrial fibrillation of recent onset. Minerva Cardioangiol. 2007 Aug;55(4):433-41.
http://www.ncbi.nlm.nih.gov/pubmed/17653020?tool=bestpractice.com
[144]Markey GC, Salter N, Ryan J. Intravenous flecainide for emergency department management of acute atrial fibrillation. J Emerg Med. 2018 Mar;54(3):320-27.
http://www.ncbi.nlm.nih.gov/pubmed/29269083?tool=bestpractice.com
The strategy for managing anticoagulation in patients presenting with new-onset AF of <48 hours' duration and with no evidence of LA thrombus is as follows:[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
If CHA₂DS₂-VASc score is 0-1, no anticoagulation is required.
If CHA₂DS₂-VAS-VASc score is ≥2, intravenous heparin (aPTT of 45-60 seconds) or subcutaneous low-molecular-weight heparin should be started before cardioversion. Once sinus rhythm is restored, the patient should preferably be started on a DOAC, unless they are not eligible for a DOAC (e.g., presence of moderate-to-severe mitral valve stenosis or mechanical prosthetic valves), or DOACs are unavailable. After the heparin is stopped, the first dose of the DOAC is usually given at the next scheduled dose time; however, local guidance should be consulted for each DOAC. If not using DOACs, start warfarin, and continue heparin until the warfarin levels are therapeutic (INR 2-3).
DOACs should not be used in patients with mechanical prosthetic valves or mitral stenosis. In such cases, warfarin is the recommended anticoagulant. The concomitant use of DOACs with heparin (including low molecular weight heparin), heparin derivatives, or warfarin is not recommended. DOACs should be used with caution in patients with renal impairment; consult prescribing information for specific guidance.
Anticoagulation should be established before cardioversion and continued for at least 4 weeks afterward, and may be required for longer in some patients.[43]Chyou JY, Barkoudah E, Dukes JW, et al. Atrial fibrillation occurring during acute hospitalization: a scientific statement from the American Heart Association. Circulation. 2023 Apr 11;147(15):e676-98.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001133
http://www.ncbi.nlm.nih.gov/pubmed/36912134?tool=bestpractice.com
[145]Sorino M, Colonna P, De Luca L, et al. Post-cardioversion transesophageal echocardiography (POSTEC) strategy with the use of enoxaparin for brief anticoagulation in atrial fibrillation patients: the multicenter POSTEC trial (a pilot study). J Cardiovasc Med (Hagerstown). 2007 Dec;8(12):1034-42.
http://www.ncbi.nlm.nih.gov/pubmed/18163016?tool=bestpractice.com
If the onset of symptoms is 48 hours or more, a 3-week regimen of uninterrupted anticoagulant therapy or imaging evaluation is recommended to rule out intracardiac thrombus before electively opting for cardioversion.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
CHA₂DS₂-VASc score also predicts risk of cardiovascular complications post cardioversion.[146]Grönberg T, Hartikainen JE, Nuotio I, et al. Anticoagulation, CHA2DS2VASc score, and thromboembolic risk of cardioversion of acute atrial fibrillation (from the FinCV study). Am J Cardiol. 2016 Apr 15;117(8):1294-8.
http://www.ncbi.nlm.nih.gov/pubmed/26892448?tool=bestpractice.com
The strategy for managing anticoagulation in these patients is as follows:
If CHA₂DS₂-VASc score is 0-1, heparin should be started, and cardioversion should be delayed until the patient is established on heparin with a target aPTT of 45-60 seconds. Following successful cardioversion, heparin can be discontinued. There is currently no evidence to support longer-term anticoagulation in patients with a score of 1 or lower. However, therapeutic decisions should be based on an individual assessment of thromboembolic versus bleeding risk; long-term anticoagulation is always required for patients with any form of cancer, irrespective of CHA₂DS₂-VASc score, even after sinus rhythm has been restored.[145]Sorino M, Colonna P, De Luca L, et al. Post-cardioversion transesophageal echocardiography (POSTEC) strategy with the use of enoxaparin for brief anticoagulation in atrial fibrillation patients: the multicenter POSTEC trial (a pilot study). J Cardiovasc Med (Hagerstown). 2007 Dec;8(12):1034-42.
http://www.ncbi.nlm.nih.gov/pubmed/18163016?tool=bestpractice.com
[147]Sulzgruber P, Wassmann S, Semb AG, et al. Oral anticoagulation in patients with non-valvular atrial fibrillation and a CHA2DS2-VASc score of 1: a current opinion of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Council on Stroke. Eur Heart J Cardiovasc Pharmacother. 2019 Jul 1;5(3):171-80.
https://academic.oup.com/ehjcvp/article/5/3/171/5497479
http://www.ncbi.nlm.nih.gov/pubmed/31119266?tool=bestpractice.com
[148]Barra S, Providência R. Anticoagulation in atrial fibrillation. Heart. 2021 Mar;107(5):419-27.
http://www.ncbi.nlm.nih.gov/pubmed/33115763?tool=bestpractice.com
[149]Apenteng PN, Virdone S, Hobbs FR, et al. Two-year outcomes of UK patients newly diagnosed with atrial fibrillation: findings from the prospective observational cohort study GARFIELD-AF. Br J Gen Pract. 2022 Feb 18 [Epub ahead of print].
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9119814
http://www.ncbi.nlm.nih.gov/pubmed/35577587?tool=bestpractice.com
If the decision is made to continue long-term anticoagulation in patients with a CHA₂DS₂-VASc score of 1, DOACs have a superior net benefit compared with vitamin K antagonists.[147]Sulzgruber P, Wassmann S, Semb AG, et al. Oral anticoagulation in patients with non-valvular atrial fibrillation and a CHA2DS2-VASc score of 1: a current opinion of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Council on Stroke. Eur Heart J Cardiovasc Pharmacother. 2019 Jul 1;5(3):171-80.
https://academic.oup.com/ehjcvp/article/5/3/171/5497479
http://www.ncbi.nlm.nih.gov/pubmed/31119266?tool=bestpractice.com
[148]Barra S, Providência R. Anticoagulation in atrial fibrillation. Heart. 2021 Mar;107(5):419-27.
http://www.ncbi.nlm.nih.gov/pubmed/33115763?tool=bestpractice.com
If CHA₂DS₂-VASc score is ≥2, all eligible patients should preferably be started on a DOAC.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
DOACs are recommended over warfarin in all cases other than for mitral stenosis and mechanical heart valve.[28]Peters NS, Schilling RJ, Kanagaratnam P, et al. Atrial fibrillation: strategies to control, combat, and cure. Lancet. 2002 Feb 16;359(9306):593-603.
http://www.ncbi.nlm.nih.gov/pubmed/11867130?tool=bestpractice.com
If warfarin is used as an oral anticoagulant, the target INR should be established for 3-4 weeks before cardioversion is attempted.[28]Peters NS, Schilling RJ, Kanagaratnam P, et al. Atrial fibrillation: strategies to control, combat, and cure. Lancet. 2002 Feb 16;359(9306):593-603.
http://www.ncbi.nlm.nih.gov/pubmed/11867130?tool=bestpractice.com
DOACs should not be used in patients with mechanical prosthetic valves and mitral stenosis.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
The concomitant use of DOACs with heparin (including low molecular weight heparin), heparin derivatives, or warfarin is contraindicated. DOACs should be used with caution in patients with renal impairment; consult prescribing information for specific guidance.
Anticoagulation should be established before cardioversion and continued for at least 4 weeks afterward, and may be required for longer in some patients.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
[43]Chyou JY, Barkoudah E, Dukes JW, et al. Atrial fibrillation occurring during acute hospitalization: a scientific statement from the American Heart Association. Circulation. 2023 Apr 11;147(15):e676-98.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001133
http://www.ncbi.nlm.nih.gov/pubmed/36912134?tool=bestpractice.com
[145]Sorino M, Colonna P, De Luca L, et al. Post-cardioversion transesophageal echocardiography (POSTEC) strategy with the use of enoxaparin for brief anticoagulation in atrial fibrillation patients: the multicenter POSTEC trial (a pilot study). J Cardiovasc Med (Hagerstown). 2007 Dec;8(12):1034-42.
http://www.ncbi.nlm.nih.gov/pubmed/18163016?tool=bestpractice.com
If there is evidence of LA thrombus on TEE, or the presence of thrombus is unknown or the duration of AF is unknown or for 48 hours or more, all eligible patients should preferably be put on a DOAC for at least 3-6 weeks; after this, imaging such as TEE should be repeated to rule out intracardiac thrombus before elective cardioversion.[1]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001193
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
[43]Chyou JY, Barkoudah E, Dukes JW, et al. Atrial fibrillation occurring during acute hospitalization: a scientific statement from the American Heart Association. Circulation. 2023 Apr 11;147(15):e676-98.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001133
http://www.ncbi.nlm.nih.gov/pubmed/36912134?tool=bestpractice.com

 ]
[Evidence A] It is, therefore, reasonable to use DOACs as first-line agents or as a subsequent replacement for warfarin in patients with AF.[1] Based on the current evidence, dabigatran is favored as a first-line agent or subsequent replacement for warfarin in suitable patients who do not have marked renal insufficiency, and who do not have mechanical prosthetic valves.[114][120]
]
[Evidence A] It is, therefore, reasonable to use DOACs as first-line agents or as a subsequent replacement for warfarin in patients with AF.[1] Based on the current evidence, dabigatran is favored as a first-line agent or subsequent replacement for warfarin in suitable patients who do not have marked renal insufficiency, and who do not have mechanical prosthetic valves.[114][120]