Early melanoma may be asymptomatic. History regarding a new or changing pigmented lesion should be obtained.
History
Questions should focus on when the lesion was first noted, although approximately 25% to 42% of melanomas arise from a pre-existing melanocytic nevus that may not be suspicious.[19]Greene MH, Clark WH Jr, Tucker MA, et al. Acquired precursors of cutaneous malignant melanoma: the familial dysplastic nevus syndrome. N Engl J Med. 1985 Jan 10;312(2):91-7.
http://www.ncbi.nlm.nih.gov/pubmed/3964923?tool=bestpractice.com
[20]Weatherhead SC, Haniffa M, Lawrence CM. Melanomas arising from naevi and de novo melanomas - does origin matter? Br J Dermatol. 2007 Jan;156(1):72-6.
http://www.ncbi.nlm.nih.gov/pubmed/17199569?tool=bestpractice.com
[21]Shitara D, Nascimento MM, Puig S, et al. Nevus-associated melanomas: clinicopathologic features. Am J Clin Pathol. 2014 Oct;142(4):485-91.
https://academic.oup.com/ajcp/article/142/4/485/1766613
http://www.ncbi.nlm.nih.gov/pubmed/25239415?tool=bestpractice.com
Another useful inquiry regards the change in size, shape, coloration, or ulceration of the lesion. If the lesion is pruritic or has bled, suspicion should be high.[50]Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004 Dec 8;292(22):2771-6.
http://www.ncbi.nlm.nih.gov/pubmed/15585738?tool=bestpractice.com
Growths with no history of a pigmented lesion are described as nodular, amelanotic, or desmoplastic melanoma.
Certain markers help identify patients at higher risk:[15]Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014 May;70(5):847-57.
http://www.ncbi.nlm.nih.gov/pubmed/24629998?tool=bestpractice.com
[17]Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005 Sep;41(14):2040-59.
http://www.ncbi.nlm.nih.gov/pubmed/16125929?tool=bestpractice.com
[33]English DR, Armstrong BK, Kricker A, et al. Sunlight and cancer. Cancer Causes Control. 1997 May;8(3):271-83.
http://www.ncbi.nlm.nih.gov/pubmed/9498892?tool=bestpractice.com
[35]Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999 Apr 29;340(17):1341-8.
http://www.ncbi.nlm.nih.gov/pubmed/10219070?tool=bestpractice.com
[36]Elwood JM, Jospson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997 Oct 9;73(2):198-203.
http://www.ncbi.nlm.nih.gov/pubmed/9335442?tool=bestpractice.com
[39]van der Leest RJ, Liu L, Coebergh JW, et al. Risk of second primary in situ and invasive melanoma in a Dutch population-based cohort: 1989-2008. Br J Dermatol. 2012 Dec;167(6):1321-30.
http://www.ncbi.nlm.nih.gov/pubmed/22759226?tool=bestpractice.com
[40]Greene MH, Clark WH Jr, Tucker MA, et al. High risk of malignant melanoma in melanoma-prone families with dysplastic nevi. Ann Intern Med. 1985 Apr;102(4):458-65.
http://www.ncbi.nlm.nih.gov/pubmed/3977193?tool=bestpractice.com
[41]Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005 Jan;41(1):28-44.
https://www.ejcancer.com/article/S0959-8049(04)00832-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/15617989?tool=bestpractice.com
[42]Kanzler MH, Mraz-Gernhard S. Primary cutaneous malignant melanoma and its precursor lesions: diagnostic and therapeutic overview. J Am Acad Dermatol. 2001 Aug;45(2):260-76.
http://www.ncbi.nlm.nih.gov/pubmed/11464189?tool=bestpractice.com
[44]Swerdlow AJ, English JS, Qiao Z. The risk of melanoma in patients with congenital nevi: a cohort study. J Am Acad Dermatol. 1995 Apr;32(4):595-9.
http://www.ncbi.nlm.nih.gov/pubmed/7896948?tool=bestpractice.com
[45]Olsen CM, Knight LL, Green AC. Risk of melanoma in people with HIV/AIDS in the pre- and post-HAART eras: a systematic review and meta-analysis of cohort studies. PLoS One. 2014 Apr 16;9(4):e95096.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3989294
http://www.ncbi.nlm.nih.gov/pubmed/24740329?tool=bestpractice.com
[46]Robbins HA, Clarke CA, Arron ST, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol. 2015 Nov;135(11):2657-65.
https://www.jidonline.org/article/S0022-202X(15)41852-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/26270022?tool=bestpractice.com
[47]Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241-50.
http://www.ncbi.nlm.nih.gov/pubmed/3545087?tool=bestpractice.com
[51]Psaty EL, Scope A, Halpern AC, et al. Defining the patient at high risk for melanoma. Int J Dermatol. 2010 Apr;49(4):362-76.
https://onlinelibrary.wiley.com/doi/10.1111/j.1365-4632.2010.04381.x/full
http://www.ncbi.nlm.nih.gov/pubmed/20465687?tool=bestpractice.com
Personal or family history of melanoma
Personal history of skin cancer (including actinic damage)
Fitzpatrick skin type I or II (white skin)
Light eye color
High freckle density
Red or blond hair
Prior sun bed use
Childhood history of sunburns
Large number of melanocytic nevi
Presence of atypical melanocytic nevi (previously called dysplastic nevi)
Presence of large (>20 cm) congenital melanocytic nevi
Genetic syndromes with skin cancer predisposition (e.g., xeroderma pigmentosum)
Immunosuppression (i.e., immunosuppressive medications, history of extensive psoralen and ultraviolet A [PUVA] therapy, or HIV).
Constitutional symptoms such as cough, weight loss, fatigue, night sweats, and headache may be symptoms of systemic metastasis in a patient with a history of melanoma.
[Figure caption and citation for the preceding image starts]: Fitzpatrick skin typeCreated by the BMJ Knowledge Centre [Citation ends].
Physical examination
Patients should receive a complete physical examination of the entire surface of the skin, including the scalp and mucous membranes.
The American Cancer Society ABCD signs of melanoma mnemonic is useful for both physicians and patients in the early detection of melanoma.[52]McGovern TW, Litaker MS. Clinical predictors of malignant pigmented lesions. A comparison of the Glasgow seven-point checklist and the American Cancer Society's ABCDs of pigmented lesions. J Dermatol Surg Oncol. 1992 Jan;18(1):22-6.
http://www.ncbi.nlm.nih.gov/pubmed/1740563?tool=bestpractice.com
The addition of evolving (letter E added to the ABCD mnemonic) has been proposed to increase the sensitivity and specificity of diagnosis using the ABCD rule.[50]Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004 Dec 8;292(22):2771-6.
http://www.ncbi.nlm.nih.gov/pubmed/15585738?tool=bestpractice.com
Asymmetry of the lesion
Border irregularity
Color variability
Diameter >6 mm
Evolution.
A specificity of 0.88 and a sensitivity of 0.73 for melanoma have been reported if 2 out of 3 of the following characteristics are noted: irregular outline, diameter >6 mm, and color variegation.[52]McGovern TW, Litaker MS. Clinical predictors of malignant pigmented lesions. A comparison of the Glasgow seven-point checklist and the American Cancer Society's ABCDs of pigmented lesions. J Dermatol Surg Oncol. 1992 Jan;18(1):22-6.
http://www.ncbi.nlm.nih.gov/pubmed/1740563?tool=bestpractice.com
In addition to the ABCDEs of melanoma, any melanocytic lesion that is atypical in appearance with respect to the patient's surrounding moles should raise suspicion. This finding has been termed the "ugly duckling sign."[50]Abbasi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004 Dec 8;292(22):2771-6.
http://www.ncbi.nlm.nih.gov/pubmed/15585738?tool=bestpractice.com
[53]Grob JJ, Bonerandi JJ. The ugly duckling sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998 Jan;134(1):103-4.
http://www.ncbi.nlm.nih.gov/pubmed/9449921?tool=bestpractice.com
Early melanoma lesions, especially those involving sun-exposed and acral sites, may be completely macular.[Figure caption and citation for the preceding image starts]: Superficial spreading melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].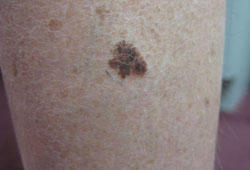 However, with progression they usually develop a papular or nodular component.[Figure caption and citation for the preceding image starts]: Nodular melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].
However, with progression they usually develop a papular or nodular component.[Figure caption and citation for the preceding image starts]: Nodular melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].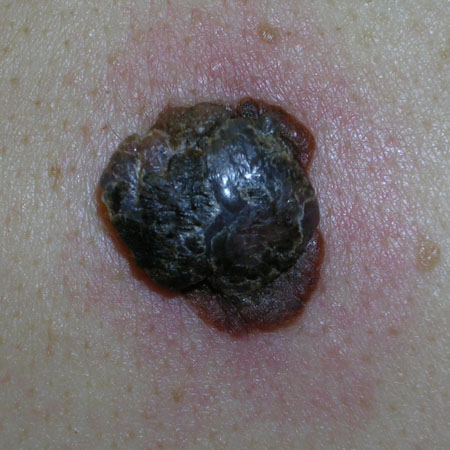
Melanomas with atypical features, such as those lacking pigment (amelanotic melanomas) or those resembling seborrheic keratoses, can be difficult to diagnose without a high index of suspicion.
Nodular melanomas tend to grow rapidly and often do not have any ABCD criteria.[54]Kalkhoran S, Milne O, Zalaudek I, et al. Historical, clinical, and dermoscopic characteristics of thin nodular melanoma. Arch Dermatol. 2010 Mar;146(3):311-8.
https://jamanetwork.com/journals/jamadermatology/fullarticle/209659
http://www.ncbi.nlm.nih.gov/pubmed/20231503?tool=bestpractice.com
In the setting of pigmented bands in the nail bed and matrix (melanonychia striata), extension of pigment into the proximal or lateral nail fold is known as Hutchinson sign and is concerning for melanoma.[55]Ronger S, Touzet S, Ligeron C, et al. Dermoscopic examination of nail pigmentation. Arch Dermatol. 2002 Oct;138(10):1327-33.
http://www.ncbi.nlm.nih.gov/pubmed/12374538?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Subungual melanoma in situFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].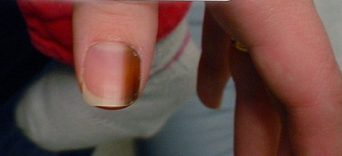
Subcutaneous masses between the skin lesion and the draining lymph node basin should raise clinical suspicion of in-transit (intralymphatic) metastases. Fixed lymphadenopathy is concerning for regional nodal metastasis.
Dermoscopy
In the UK, it is recommended that any lesion that is suspected for melanoma on physical examination should be further evaluated with dermoscopy.[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
[57]Koblinski JE, Ahrns HT, Morse MJ, et al. Dermoscopy for the identification of amelanotic acral melanoma. J Am Podiatr Med Assoc. 2022 Mar 16;112(1):20-184.
http://www.ncbi.nlm.nih.gov/pubmed/36459055?tool=bestpractice.com
[58]Williams NM, Rojas KD, Reynolds JM, et al. Assessment of diagnostic accuracy of dermoscopic structures and patterns used in melanoma detection: a systematic review and meta-analysis. JAMA Dermatol. 2021 Sep 1;157(9):1078-88.
https://jamanetwork.com/journals/jamadermatology/fullarticle/2782522
http://www.ncbi.nlm.nih.gov/pubmed/34347005?tool=bestpractice.com
[59]Lan J, Wen J, Cao S, et al. The diagnostic accuracy of dermoscopy and reflectance confocal microscopy for amelanotic/hypomelanotic melanoma: a systematic review and meta-analysis. Br J Dermatol. 2020 Aug;183(2):210-9.
http://www.ncbi.nlm.nih.gov/pubmed/31747045?tool=bestpractice.com
In the US, skin biopsy is the preferred method of further evaluation of suspicious lesions.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
In dermoscopic evaluation, the skin lesion is usually covered with an immersion fluid. The skin lesion is then inspected using a handheld magnifying lens attached to a light source. Dermoscopes with a polarized light source do not require an immersion liquid.
The most important application of dermoscopy is distinguishing melanoma from benign melanocytic lesions. However, the technique is also useful in distinguishing melanocytic lesions from nonmelanocytic pigmented lesions such as seborrheic keratoses, pigmented basal cell carcinomas, and vascular lesions.
[Figure caption and citation for the preceding image starts]: Dermoscopy: the most important application of dermoscopy is distinguishing melanoma from benign melanocytic lesions© DermNet New Zealand; used with permission [Citation ends].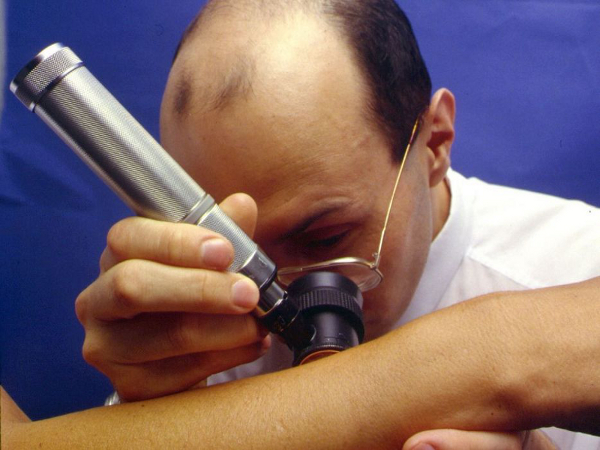
The use of dermoscopy by trained individuals results in increased diagnostic accuracy compared with naked-eye exam alone.[60]Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008 Sep;159(3):669-76.
http://www.ncbi.nlm.nih.gov/pubmed/18616769?tool=bestpractice.com
Utilizing dermoscopy, the nonabsolute sensitivity for diagnosing melanoma increased by 19% (83.2% with dermoscopy versus 69.6% without dermoscopy).[61]Menzies SW, Zalaudek I. Why perform dermoscopy? The evidence for its role in the routine management of pigmented skin lesions. Arch Dermatol. 2006 Sep;142(9):1211-2.
http://www.ncbi.nlm.nih.gov/pubmed/16983009?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Key dermoscopic features of melanoma: (A) Melanoma presenting with atypical globules and dots of different sizes and shapes (yellow arrows), patches of atypical network (blue arrowhead) and a blue-white veil (blue arrow). (B) Melanoma with diffuse polymorphous vasculature, consisting of serpentine, dotted, and glomerular vessels, can be found throughout the lesion (yellow arrowheads); in addition, patches of atypical network (blue arrowheads) are seen. (C) Superficial spreading melanoma with pseudopods distributed asymmetrically around the lesion (black arrowheads). (D) Melanoma with the regression structure blue-gray peppering (black star); shiny white lines are also seen throughout the entire lesion (red arrows) along with a central blue-white veil (red arrowhead)Wolner ZJ et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017 Oct;35(4):417-37; used with permission [Citation ends].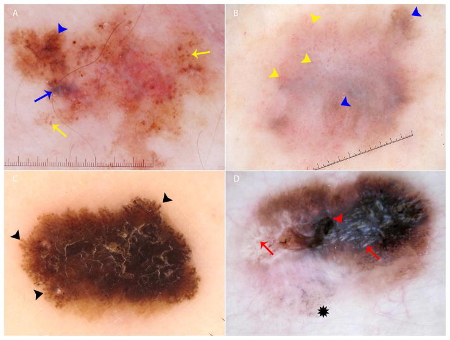
Skin biopsy
Histopathologic analysis of a new or changing atypical pigmented lesion is essential in the diagnosis. Any suspicious lesion should be biopsied. The excision of a pigmented lesion enables the dermatopathologist to accurately diagnose melanoma, and influences prognosis and the extent of further surgery or other treatment.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
The ideal management is a complete full-thickness excision of the lesion with a 1-3 mm margin.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
[62]Amin MB, Edge S, Greene F, et al, eds. AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017. Full-thickness incisional or punch biopsy of clinically thickest or most atypical portion of lesion is acceptable and may be preferred in certain anatomic areas (e.g., palm/sole, digit, face, ear) or for very large lesions. Multiple "scouting" biopsies may help guide management for very large lesions.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
Skin biopsy confirms the diagnosis of melanoma and provides prognostic information. Prognostic indicators include tumor thickness measured as Breslow thickness (depth of invasion measured in millimeters from the top of the granular cell layer to the point of deepest tumor penetration in the dermis or subcutis), ulceration, mitotic count, lymphovascular invasion, and microscopic satellites.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
[63]Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970 Nov;172(5):902-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1397358
http://www.ncbi.nlm.nih.gov/pubmed/5477666?tool=bestpractice.com
An unfavorable prognosis is associated with greater depth of invasion, ulceration, increased mitotic count, vascular invasion, regression, and the presence of microscopic satellites.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
Immunohistochemistry
Immunohistochemistry is increasingly used to interpret biopsies that are not easily classified based on histopathologic features.[64]Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20(11):1366-79.
https://www.tandfonline.com/doi/full/10.1080/15384047.2019.1640032
http://www.ncbi.nlm.nih.gov/pubmed/31366280?tool=bestpractice.com
[65]Dinehart MS, Dinehart SM, Sukpraprut-Braaten S, et al. Immunohistochemistry utilization in the diagnosis of melanoma. J Cutan Pathol. 2020 May;47(5):446-50.
http://www.ncbi.nlm.nih.gov/pubmed/31955450?tool=bestpractice.com
It can help to distinguish melanocytic lesions from tumors with different origin, and benign from malignant melanocytic lesions.[66]Ivan D, Prieto VG. Use of immunohistochemistry in the diagnosis of melanocytic lesions: applications and pitfalls. Future Oncol. 2010 Jul;6(7):1163-75.
http://www.ncbi.nlm.nih.gov/pubmed/20624128?tool=bestpractice.com
Melanocytic markers that can be used in the immunohistochemical analysis include Melan-A/MART-1, S-100, MITF, tyrosinase, HMB45, SOX10, and PRAME.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
[64]Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20(11):1366-79.
https://www.tandfonline.com/doi/full/10.1080/15384047.2019.1640032
http://www.ncbi.nlm.nih.gov/pubmed/31366280?tool=bestpractice.com
[67]Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: diagnostics - update 2022. Eur J Cancer. 2022 Jul:170:236-55.
https://www.ejcancer.com/article/S0959-8049(22)00152-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35570085?tool=bestpractice.com
[68]Lezcano C, Jungbluth AA, Busam KJ. Immunohistochemistry for PRAME in dermatopathology. Am J Dermatopathol. 2023 Nov 1;45(11):733-47.
http://www.ncbi.nlm.nih.gov/pubmed/37856737?tool=bestpractice.com
Genomic analysis of melanoma
Serves to identify common genetic mutations associated with melanoma. Increasingly, gene panels are performed to test for mutations of interest.
BRAF mutational analysis
Approximately 40% of melanomas contain an activating mutation in the BRAF gene, most commonly at position V600. Valine to glutamate (V600E) accounts for 75% to 90% of BRAF mutations within melanoma. Other less common mutations include valine to lysine (V600K) in 10% to 30% of BRAF mutations, and valine to arginine (V600R) in 1% to 3%. These mutations lead to constitutive activation of the mitogen-activated protein kinase signaling pathway.[26]Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011 Apr 1;29(10):1239-46.
http://ascopubs.org/doi/full/10.1200/jco.2010.32.4327
http://www.ncbi.nlm.nih.gov/pubmed/21343559?tool=bestpractice.com
[27]Jakob JA, Bassett RL Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012 Aug 15;118(16):4014-23.
http://onlinelibrary.wiley.com/doi/10.1002/cncr.26724/full
http://www.ncbi.nlm.nih.gov/pubmed/22180178?tool=bestpractice.com
[28]Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res. 2012 Jun 15;18(12):3242-9.
https://clincancerres.aacrjournals.org/content/18/12/3242.long
http://www.ncbi.nlm.nih.gov/pubmed/22535154?tool=bestpractice.com
Recommendations for BRAF mutational analysis vary by country. It is generally performed in patients who will require systemic therapy, and in those at high risk of recurrence for whom future BRAF-directed therapy may be appropriate.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
In the UK, immunohistochemistry is recommended as the initial test for BRAF V600E if available.[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
This is rapid, with high sensitivity and specificity for BRAF V600E.[69]Long GV, Wilmott JS, Capper D, et al. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol. 2013 Jan;37(1):61-5.
http://www.ncbi.nlm.nih.gov/pubmed/23026937?tool=bestpractice.com
If immunohistochemistry is inconclusive or negative, a different BRAF genetic test should be tried.[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
An automated molecular assay to identify BRAF mutations at position V600 is available, but it only readily detects the V600E mutation.[70]Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011 Jun 30;364(26):2507-16.
http://www.nejm.org/doi/full/10.1056/NEJMoa1103782#t=article
http://www.ncbi.nlm.nih.gov/pubmed/21639808?tool=bestpractice.com
Several other platforms, including pyrosequencing, next generation sequencing, and Sanger sequencing, detect all known V600 mutations.
All patients who test positive for any BRAF V600 mutation are suitable for BRAF/MEK inhibitor therapy.
NRAS mutation analysis
NRAS mutations activate the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways, thereby promoting tumor proliferation and survival, are found in approximately 15% to 25% of melanomas and are associated with rapidly progressing disease.[25]Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol. 2011 Apr;164(4):776-84.
http://www.ncbi.nlm.nih.gov/pubmed/21166657?tool=bestpractice.com
[71]Gutiérrez-Castañeda LD, Nova JA, Tovar-Parra JD. Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: a systemic review. Melanoma Res. 2020 Feb;30(1):62-70.
https://journals.lww.com/melanomaresearch/Fulltext/2020/02000/Frequency_of_mutations_in_BRAF,_NRAS,_and_KIT_in.6.aspx
http://www.ncbi.nlm.nih.gov/pubmed/31274706?tool=bestpractice.com
More than 80% of NRAS mutations involve a point mutation leading to the substitution of glutamine to leucine at position 61, and in melanoma are generally mutually exclusive to BRAF mutations.
Detection methods include post-polymerase chain reaction high resolution melt analysis.
CDKN2A mutation analysis
Mutations in the CDKN2A gene on chromosome 9 have been found in families with the atypical nevus syndrome and in 39% of melanoma-prone families.[18]Soura E, Eliades PJ, Shannon K, et al. Hereditary melanoma: update on syndromes and management: genetics of familial atypical multiple mole melanoma syndrome. J Am Acad Dermatol. 2016 Mar;74(3):395-407; quiz 408-10.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4761105
http://www.ncbi.nlm.nih.gov/pubmed/26892650?tool=bestpractice.com
[38]Goldstein AM, Chan M, Harland M, et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet. 2007 Feb;44(2):99-106.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2598064
http://www.ncbi.nlm.nih.gov/pubmed/16905682?tool=bestpractice.com
Next generation sequencing or single gene assays may be used for molecular testing in the research setting.
If there are existing genetic test results, do not order a duplicate test unless there is uncertainty about the existing result, for example the result is inconsistent with the patient’s clinical presentation or the test methodology has changed.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
[72]American College of Medical Genetics and Genomics. Five things physicians and patients should question. Choosing Wisely, an initiative of the ABIM Foundation. 2021 [internet publication].
https://web.archive.org/web/20230326143738/https://www.choosingwisely.org/societies/american-college-of-medical-genetics-and-genomics
Clinical staging of melanoma: American Joint Committee on Cancer TNM staging system (8th Edition)
The American Joint Committee on Cancer (AJCC) staging system describes the extent of disease based on the following anatomic factors: size and extent of the primary tumor (T); regional lymph node involvement (N); and presence or absence of distant metastases (M).[62]Amin MB, Edge S, Greene F, et al, eds. AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017. Nonanatomic prognostic factors (e.g., tumor grade, biomarkers) may be used to supplement the staging of certain cancers.
In general, cutaneous melanoma is categorized as:[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
Localized disease (no evidence of metastases, stage I-II)
Regional disease (stage III)
Distant metastatic disease (stage IV).
Imaging
Ultrasound of the lymph node basin may be performed before sentinel lymph node biopsy, if the physical examination of the lymph nodes is equivocal. Ultrasound is not needed for clinically suspicious lymph nodes, because biopsy is indicated. Any suspicious lymph nodes identified on ultrasound should be biopsied.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
Baseline cross-sectional imaging
Guideline recommendations for use of baseline cross-sectional imaging differ.
In the US, baseline cross-sectional staging imaging should be considered for patients with stage IIIa melanoma (sentinel lymph node positive), and is indicated for all patients with stage IIIb, IIIc, IIId, and IV melanoma. Imaging studies should include the chest, abdomen, and pelvis, plus the neck if clinically indicated. CT or fluorodeoxyglucose positron emission tomography/computed tomography (FDG-CT/PET) may be used.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
FDG-CT/PET has the highest specificity, sensitivity, and diagnostic odds ratio for distant metastases.[73]Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011 Jan 19;103(2):129-42.
https://academic.oup.com/jnci/article/103/2/129/2568844
http://www.ncbi.nlm.nih.gov/pubmed/21081714?tool=bestpractice.com
For stages IIIb, IIIc, and IIId brain imaging for baseline staging may be indicated prior to initiation of therapy, and is recommended for all patients with stage IV melanoma.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
Imaging should also be performed to evaluate any symptoms or signs suggestive of possible metastases (e.g., headache) if present, at any stage of disease.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
In the UK, whole-body and brain contrast-enhanced computed tomography (CE-CT) should be considered for people with stage IIB melanoma.[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
Staging with CE-CT should be offered to all patients with IIC to IV melanoma. If available, a brain magnetic resonance imaging (MRI) can be used instead of a brain CE-CT.[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
A whole-body and brain MRI, instead of CE-CT should be offered to:[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
For people with stage IIIC to IV melanoma and one of the following risk factors, a staging brain MRI, instead of a CE-CT can be considered:[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
If adjuvant treatment is begun longer than 8 weeks after initial staging, a repeat staging scan should be considered before starting adjuvant treatment.[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
Routine imaging is not recommended for people with stage IA melanoma, and should not be considered before sentinel lymph node biopsy (SLNB) unless lymph node or distant metastases are suspected.[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
One European guideline recommends baseline brain imaging with magnetic resonance imaging (MRI) with intravenous contrast or CT should be considered for patients with stage IIC melanoma with a poor prognosis, and is indicated for all patients with stage III to IV melanoma.[67]Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: diagnostics - update 2022. Eur J Cancer. 2022 Jul:170:236-55.
https://www.ejcancer.com/article/S0959-8049(22)00152-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35570085?tool=bestpractice.com
MRI with intravenous contrast is the preferred method for brain imaging, as it is better at detecting brain metastases than CT scan.[67]Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: diagnostics - update 2022. Eur J Cancer. 2022 Jul:170:236-55.
https://www.ejcancer.com/article/S0959-8049(22)00152-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35570085?tool=bestpractice.com
Sentinel lymph node biopsy
Sentinel lymph node biopsy (SLNB) is based on the concept that a tumor will drain to a particular first lymph node within a lymph node basin. There may be multiple draining lymph node basins and multiple sentinel nodes, depending on individual lymphatic drainage patterns.
A radiotracer or a blue dye is injected intradermally at the site of the primary lesion (before wide local excision [WLE]) to identify the sentinel node.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
SLNB may be performed for selected patients with stage I and stage II melanoma. It permits accurate staging of patients with no clinical or radiologic evidence of nodal metastases, and provides prognostic information.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
NCCN guidelines advise that SLNB should be discussed with patients with stage IB or II disease with the following considerations:[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
Tumor thickness <0.8 mm with ulceration
Tumor thickness 0.8 to 1.0 mm with or without ulceration
Tumor thickness >0.5 mm who have additional adverse prognostic features (e.g., age ≤42 years, head/neck location, lymphovascular invasion, and/ or mitotic index ≥2/mm²)
The probability of a positive SLNB is 5% to 10% for these patients, with an increased risk for patients with multiple adverse prognostic features.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
SLNB is not generally recommended for patients with tumors <0.8 mm thick without ulceration or adverse prognostic features, unless there is significant uncertainty about the adequacy of microstaging (e.g., positive deep margins or limited sampling of a larger lesion). The risk of positive SLNB in these patients is <5%.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
UK guidelines recommend considering SLNB as a staging (rather than therapeutic) procedure for patients with a Breslow thickness of 0.8 to 1.0 mm, with at least one of following features:[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
For people without adverse prognostic features, SLNB should be considered with a Breslow thickness of greater than 1.0 mm.[56]National Institute for Health and Care Excellence. Melanoma: assessment and management. Jul 2022 [internet publication].
https://www.nice.org.uk/guidance/NG14
Consider delaying SLNB for pregnant women until postpartum.
One phase 3 trial reported that SLNB does not improve overall survival for patients with melanoma, but subgroup analysis of the Multicenter Selective Lymphadenectomy Trial (MSLT)-I showed that SLNB improves the 10-year distant disease-free survival for patients with melanomas between 1.2 mm and 3.5 mm in thickness.[74]Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014 Feb 13;370(7):599-609.
http://www.ncbi.nlm.nih.gov/pubmed/24521106?tool=bestpractice.com
[  ]
How does sentinel lymph node biopsy plus dissection compare with observation in people with localized primary cutaneous melanoma?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.848/fullShow me the answer However, subsequent evidence from a systematic review demonstrated that SLNB is associated with improved overall survival in patients with head and neck cutaneous melanoma.[75]Zhang Y, Liu C, Wang Z, et al. Sentinel lymph node biopsy in head and neck cutaneous melanomas: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2021 Feb 5;100(5):e24284.
https://journals.lww.com/md-journal/fulltext/2021/02050/sentinel_lymph_node_biopsy_in_head_and_neck.59.aspx
http://www.ncbi.nlm.nih.gov/pubmed/33592872?tool=bestpractice.com
]
How does sentinel lymph node biopsy plus dissection compare with observation in people with localized primary cutaneous melanoma?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.848/fullShow me the answer However, subsequent evidence from a systematic review demonstrated that SLNB is associated with improved overall survival in patients with head and neck cutaneous melanoma.[75]Zhang Y, Liu C, Wang Z, et al. Sentinel lymph node biopsy in head and neck cutaneous melanomas: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2021 Feb 5;100(5):e24284.
https://journals.lww.com/md-journal/fulltext/2021/02050/sentinel_lymph_node_biopsy_in_head_and_neck.59.aspx
http://www.ncbi.nlm.nih.gov/pubmed/33592872?tool=bestpractice.com
Laboratory tests
Serum lactate dehydrogenase (LDH) has prognostic value in patients with stage IV melanoma and has been incorporated into the AJCC staging system for melanoma.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
[62]Amin MB, Edge S, Greene F, et al, eds. AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017.[76]Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019 Jan;80(1):208-50.
https://www.jaad.org/article/S0190-9622(18)32588-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30392755?tool=bestpractice.com
Patients with distant metastases and elevated LDH are in the highest risk category.[14]National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: melanoma - cutaneous [internet publication].
https://www.nccn.org/guidelines/category_1
Some evidence suggests that LDH may serve as a potential biomarker to identify patients who would benefit from anti-PD 1/PD-L1 treatment.[77]Xu J, Zhao J, Wang J, et al. Prognostic value of lactate dehydrogenase for melanoma patients receiving anti-PD-1/PD-L1 therapy: a meta-analysis. Medicine (Baltimore). 2021 Apr 9;100(14):e25318.
https://journals.lww.com/md-journal/fulltext/2021/04090/prognostic_value_of_lactate_dehydrogenase_for.50.aspx
http://www.ncbi.nlm.nih.gov/pubmed/33832106?tool=bestpractice.com
No blood work is usually necessary in patients with other stages of melanoma unless they have systemic symptoms.

 However, with progression they usually develop a papular or nodular component.[Figure caption and citation for the preceding image starts]: Nodular melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].
However, with progression they usually develop a papular or nodular component.[Figure caption and citation for the preceding image starts]: Nodular melanomaFrom the personal collection of Dr Hobart Walling and Dr Brian Swick. [Citation ends].



 ]
However, subsequent evidence from a systematic review demonstrated that SLNB is associated with improved overall survival in patients with head and neck cutaneous melanoma.[75]
]
However, subsequent evidence from a systematic review demonstrated that SLNB is associated with improved overall survival in patients with head and neck cutaneous melanoma.[75]