Etiology
Endometrial hyperplasia
Endometrial hyperplasia is characterized by proliferation of endometrial glands resulting in a greater gland-to-stroma ratio than is observed in normal endometrium. It has been associated with progression to adenocarcinoma in some studies.[31][32][33]
Endometrial hyperplasia commonly results from chronic estrogen stimulation unopposed by the counterbalancing effects of progesterone.[31]
Complex hyperplasia with cytologic atypia is termed endometrial intraepithelial neoplasia (EIN).[32][Figure caption and citation for the preceding image starts]: Epithelial in situ neoplasia arising in proliferative endometrium.(photomicrograph, hematoxylin and eosin stain)From the collection of George Mutter MD, Division of Women's and Perinatal Pathology, Brigham and Women's Hospital, Harvard Medical School [Citation ends].

With the increasing use of progesterone receptor modulators (PRMs) such as mifepristone (RU486) for the management of endometriosis and uterine leiomyoma, the term "PRM-associated endometrial changes" has been proposed; these histologic changes in the endometrium should not be confused with EIN.[33]
Unopposed endogenous estrogenic stimulation of the endometrium
Seen in chronic anovulation, which is a feature of polycystic ovary syndrome.
In postmenopausal women, estrogens are primarily produced through peripheral aromatization of androgens, particularly androstenedione, in adipocytes. Obesity increases the production rate of estrogens by this mechanism.
Sex cord stromal tumors of the ovary, such as granulosa cell tumors, are also a source of endogenous estradiol.
Unopposed exogenous estrogenic stimulation of the endometrium
Exogenous estrogen therapy (e.g., hormone replacement therapy) in premenopausal and postmenopausal women is associated with endometrial hyperplasia and endometrial cancer.[31][34][35]
Familial cancer syndromes
Lynch syndrome (also known as hereditary nonpolyposis colorectal cancer) is associated with mutations in DNA mismatch repair (MMR) genes (including MSH2, MLH1, MSH6, and PMS2) and deletions in the epithelial cellular adhesion molecule (EPCAM) gene.[36][37]
Family history of colorectal, endometrial, and/or ovarian cancer is often observed.[38][39]
Cowden syndrome, related to mutation in the PTEN tumor suppressor gene. Carriers have an increased risk for endometrial, breast, thyroid, colorectal, and renal cancer.[40]
Pathophysiology
There are a number of pathophysiologic factors that may be associated with endometrial cancer.
Estrogen: the ability of estrogen to function as a mitogen is clear, while its ability to act as a mutagen in stimulating cellular division and organ growth is controversial. The former effects seem to occur via stimulation of the transcription of genes for cyclin D, proto-oncogenes, growth factors, and growth factor receptors. Estrogen may affect the expression of genes, leading to altered regulation of cellular signals in the development of endometrial hyperplasia. Phosphatase and tensin homologue (PTEN) gene mutation with loss of expression of the PTEN protein is an early event in this progression, while mutations of ras and mismatch repair genes occur later.[40][41][42][43]
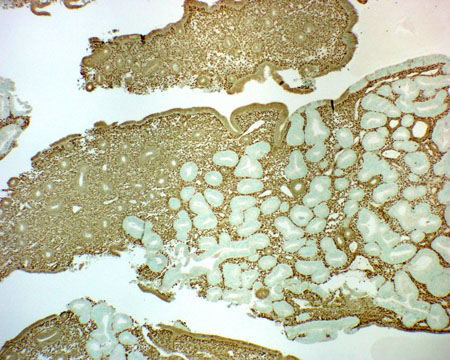
PTEN tumor suppressor gene mutation: paraffin tissue immunohistochemistry with anti-PTEN antibody shows that over half of endometrioid endometrial adenocarcinomas, and their precursor EIN lesions, have lost PTEN protein.[44][45][Figure caption and citation for the preceding image starts]: Low-grade adenocarcinoma arising in proliferative endometrium, stained using immunohistochemistry (brown stain) for phosphatase and tensin homolog protein; note the negatively stained (nonbrown) neoplastic glands (normal epithelium usually stains brown)From the collection of George Mutter MD, Division of Women's and Perinatal Pathology, Brigham and Women's Hospital, Harvard Medical School [Citation ends].
K-ras gene mutation: endometrioid adenocarcinomas, which are estrogen-dependent (and account for 80% of all endometrial cancers), contain K-ras mutations in 24% of cases.[46]
Microsatellite instability and mismatch repair (MMR) genes: endometrioid adenocarcinomas, which are estrogen-dependent, show microsatellite instability in 20% to 30% of cases.[47][48] Germline mutations in the MMR genes MLH1, MSH2, MSH6, and PMS2 can lead to the development of Lynch syndrome (hereditary nonpolyposis colorectal cancer). Deletions in the epithelial cellular adhesion molecule (EPCAM) gene can cause Lynch syndrome by silencing MSH2 expression. These mutations confer a strong susceptibility to cancer, including endometrial, ovarian, and colorectal cancer.[40][41][49]
p53 gene mutation: mutation of the p53 tumor suppressor gene is not found in endometrial hyperplasia, but can be detected in 20% of cases of endometrioid carcinoma and in more than 90% of serous tumors of the endometrium (which are estrogen-independent and arise from atrophic, rather than hyperplastic, endometrium).[13]
HER-2/neu gene: overexpression or amplification of HER2 (encoded by the ERBB2 gene) in uterine serous carcinoma is associated with a poor prognosis. Uterine serous carcinoma, a biologically distinct subtype of endometrial cancer, is characterized by six major molecular genetic alterations including: TP53 mutation, PIK3CA activating mutation/amplification, ERBB2 amplification, CCNE1 amplification, FBXW7 inactivating mutation/deletion, and PPP2R1A inactivating mutation.[50]
Genome-wide association studies have identified additional candidate genes, which encode negative regulators of oncogenic signal transduction proteins, on chromosomes 12 and 17.[51]
Classification
WHO classification of tumors of the uterine corpus[1]
Endometrial epithelial tumors and precursors
Precursor lesions
Endometrial hyperplasia without atypia
Endometrial atypical hyperplasia/endometrioid intraepithelial neoplasia
Endometrial carcinomas
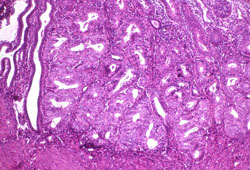
Endometrioid carcinoma of the uterine corpus: the most common subtype of endometrial cancer, present in up to 90% of cases, with histology showing recognizable malignant glands and glandular cells in the better-differentiated (low-grade) forms[2][Figure caption and citation for the preceding image starts]: Histologic subtype: endometrioid endometrial adenocarcinoma, the commonest subtype; diagnosed on dilation and curettage in a patient presenting with postmenopausal bleeding (photomicrograph, hematoxylin and eosin stain)Courtesy of Professor Robert H. Young, Department of Pathology, Massachusetts General Hospital [Citation ends].
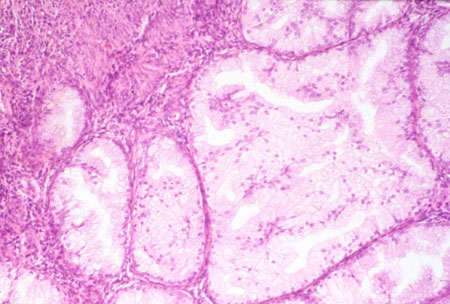
Serous carcinoma of the uterine corpus[Figure caption and citation for the preceding image starts]: Histologic subtype: uterine papillary serous carcinoma with typical small papillae and slit-like spaces (photomicrograph, hematoxylin and eosin stain)Courtesy of Professor Robert H. Young, Department of Pathology, Massachusetts General Hospital [Citation ends].

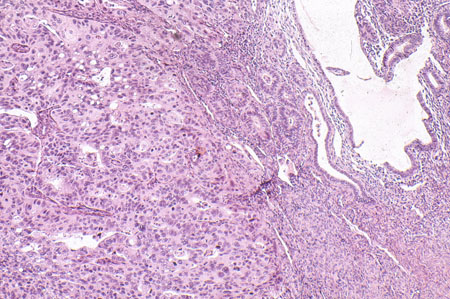
Clear cell carcinoma of the uterine corpus (1% to 6% of cases)[3][4][5][Figure caption and citation for the preceding image starts]: Histologic subtype: clear cell adenocarcinoma (photomicrograph, hematoxylin and eosin stain)Courtesy of Professor Robert H. Young, Department of Pathology, Massachusetts General Hospital [Citation ends].
Undifferentiated and dedifferentiated carcinomas of the uterine corpus
Mixed carcinoma of the uterine corpus
Other endometrial carcinomas
Carcinosarcoma of the uterine corpus
Tumor-like lesions
Endometrial polyp
Endometrial metaplasia
Arias-Stella reaction of the uterine corpus
Mesenchymal tumors of the uterus
Smooth muscle tumors
Uterine leiomyoma
Intravenous leiomyomatosis
Smooth muscle tumor of uncertain malignant potential of the uterine corpus
Metastasizing leiomyoma
Uterine leiomyosarcoma
Endometrial stromal and related tumors
Endometrial stromal sarcoma, low grade
Endometrial stromal sarcoma, high grade
Endometrial stromal nodule
Undifferentiated endometrial sarcoma
Miscellaneous mesenchymal tumors
Uterine tumor resembling ovarian sex cord tumor
Perivascular epithelioid cell tumor (PEComa)
Inflammatory myofibroblastic tumor
Other mesenchymal tumors of the uterus
Mixed epithelial and mesenchymal tumors
Adenomyoma of the uterine corpus
Atypical polypoid adenomyoma
Adenosarcoma of the uterine corpus
Miscellaneous tumors
Tumors of germ cell type
Central primitive neuroectodermal tumor/central nervous system embryonal tumor
International Federation of Gynecology and Obstetrics (FIGO) histologic grading[6][7]
Endometrial cancers are primarily classified as endometrioid versus nonendometrioid. Endometrioid cancers constitute the majority, are most commonly present as early stage grade 1-2, are often hormone dependent, and have a more favorable clinical course. Grade 3 endometrioid cancers are more mixed and generally have a less favorable prognosis.
Nonendometrioid cancers are more aggressive serous cancers, clear cell cancers, and carcinosarcomas that typically are at higher risk of early distant spread.[7]
Histopathologic grades:
GX: Grade cannot be assessed
G1: Well differentiated
G2: Moderately differentiated
G3: Poorly differentiated or undifferentiated.
Degree of differentiation is another basis for classification:
Grade 1 (G1): nonsquamous or nonmorular solid growth pattern of ≤5%[Figure caption and citation for the preceding image starts]: Grade 1 or low-grade endometrioid adenocarcinoma (right) on background of proliferative endometrium (left) (photomicrograph, hematoxylin and eosin stain)Courtesy of Professor Robert H. Young, Department of Pathology, Massachusetts General Hospital [Citation ends].

Grade 2 (G2): nonsquamous or nonmorular solid growth pattern of 6% to 50%
Grade 3 (G3): nonsquamous or nonmorular solid growth pattern of >50%. [Figure caption and citation for the preceding image starts]: Grade 3 or high-grade endometrioid adenocarcinoma on a background of atrophic endometrium (photomicrograph, hematoxylin and eosin stain)Courtesy of Professor Robert H. Young, Department of Pathology, Massachusetts General Hospital [Citation ends].
Generally, G1 and G2 are low grade and G3 is high grade.
Patient phenotype, I or II
Type I endometrial carcinoma:
Most commonly seen in the classical clinical setting of an obese patient with hyperlipidemia, hyperestrogenism, insulin resistance, and infertility
Also common in women with uterine bleeding and late onset of menopause associated with estrogen-induced endometrial hyperplasia
These lower-grade hormone receptor-positive endometrioid cancers more commonly occur in younger women.[8]
Type II endometrial carcinomas:
More typically arise in atrophic endometrium in older women
Associated with TP53 mutations
Tumor hormone receptor status[9]
Estrogen receptor (ER) and progesterone receptor (PR) status is only evaluated for potential palliative use of hormonal therapy in advanced or recurrent tumors, but does have prognostic value:
Presence of ER (ER positive)
Presence of PR (PR positive)
Clinical response to a trial of a progestin (typically medroxyprogesterone).
Tumor biomarker status
Used increasingly in order to subclassify endometrial carcinoma and for the purposes of treatment and prognosis.
Loss of the tumor suppressor phosphatase and tensin homolog (PTEN) gene has been reported in 30% to 50% of type 1 cancers, but is rarely observed in serous cancers.[11][12][13]
The proto-oncogene HER2/neu, a transmembrane growth factor receptor, is commonly amplified or over-expressed in serous cancers, and is associated with poor survival.[14][15][16][17][18]
Serous tumors also have high rates of p53 mutation, less commonly observed in type 1 and lower-grade tumors.
Commonly used biomarkers can be detected by most surgical pathology labs using immunohistochemistry on tissue sections:
PTEN[Figure caption and citation for the preceding image starts]: Low-grade adenocarcinoma arising in proliferative endometrium, stained using immunohistochemistry (brown stain) for phosphatase and tensin homolog protein; note the negatively stained (nonbrown) neoplastic glands (normal epithelium usually stains brown)From the collection of George Mutter MD, Division of Women's and Perinatal Pathology, Brigham and Women's Hospital, Harvard Medical School [Citation ends].

Mismatch-repair genes MLH1, MSH2, MSH6, and PMS2
p53
HER-2
Phospho-AKT (controversial).
The Cancer Genome Atlas (TCGA) classification[19]
TCGA research network characterized 373 endometrial carcinomas using array- and sequencing-based technologies, and proposed the following four distinct molecular subgroups of endometrial carcinomas:
POLE gene ultramutated (endometrioid carcinomas with POLE mutations)
Microsatellite instability hypermutated (endometrioid carcinomas with microsatellite instability)
Copy-number low (endometrioid carcinomas with a low degree of copy-number alterations)
Copy-number high (predominantly serous, but also serous-like endometrioid carcinomas with a high degree of copy-number alterations and frequent TP53 mutations).
Molecular profiling is increasingly preferred to morphologic classification to inform prognosis and treatment-related decisions.[7] Molecular classification is being integrated into national and international guidelines.[7]
Proactive Molecular Risk Classifier for Endometrial Cancer (ProMisE)[20]
The novel molecular classifier ProMisE assigns patients with endometrial cancer to one of four groups based on a combination of mutation and protein expression analyses. The first assessment is immunohistochemistry to determine the presence or absence of mismatch repair proteins (to identify MMR deficiency [MMR-D]), enabling a rapid referral to hereditary cancer programs.
Tumors are then assessed for POLE mutations, and finally for protein 53, resulting in four subgroups:
MMR-D - Mismatch repair (MMR) deficient
POLE EDM - Polymerase-e (POLE) exonuclease domain mutations (EDMs)
P53 wt - Protein 53 (p53) wild type (wt)
P53 abn - Protein 53 null/missense mutations.
Use of this content is subject to our disclaimer