The main goals of treatment are to limit myocardial damage by restoring myocardial blood flow as quickly as possible and to decrease subsequent remodeling, which can have deleterious effects on ventricular function and prognosis.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[87]Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165.
https://academic.oup.com/eurheartj/article/40/2/87/5079120
http://www.ncbi.nlm.nih.gov/pubmed/30165437?tool=bestpractice.com
Immediate and prompt revascularization with percutaneous coronary intervention (PCI) within 90 minutes of first presentation, or thrombolysis within 12 hours of symptom onset, can prevent or decrease myocardial damage and decrease morbidity and mortality by preventing acute complications.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[107]Redfors B, Mohebi R, Giustino G, et al. Time delay, infarct size, and microvascular obstruction after primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2021 Feb;14(2):e009879.
https://www.ahajournals.org/doi/10.1161/CIRCINTERVENTIONS.120.009879
http://www.ncbi.nlm.nih.gov/pubmed/33440999?tool=bestpractice.com
It is strongly recommended that local communities or regional areas should develop a rapid response system for treatment of STEMI. The optimal coronary revascularization strategy may not be clear for all patients with STEMI (e.g., if there is complex coronary disease and/or comorbid conditions); this is more likely to apply to older patients.[108]Damluji AA, Forman DE, Wang TY, et al. Management of acute coronary syndrome in the older adult population: a scientific statement from the American Heart Association. Circulation. 2022 Dec 12 [Epub ahead of print].
https://www.doi.org/10.1161/CIR.0000000000001112
http://www.ncbi.nlm.nih.gov/pubmed/36503287?tool=bestpractice.com
American College of Cardiology/American Heart Association guidelines recommend a patient-centered shared decision-making process for such patients, utilizing a multidisciplinary team that includes representatives from interventional cardiology, cardiac surgery, and clinical cardiology.[67]Gulati M, Levy PD, Mukherjee D, et al; Writing Committee Members. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021 Nov 30;78(22):e187-285.
https://www.jacc.org/doi/10.1016/j.jacc.2021.07.053
http://www.ncbi.nlm.nih.gov/pubmed/34756653?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[108]Damluji AA, Forman DE, Wang TY, et al. Management of acute coronary syndrome in the older adult population: a scientific statement from the American Heart Association. Circulation. 2022 Dec 12 [Epub ahead of print].
https://www.doi.org/10.1161/CIR.0000000000001112
http://www.ncbi.nlm.nih.gov/pubmed/36503287?tool=bestpractice.com
Initial management
The patient should be admitted to a unit with continuous cardiac monitoring and started on strict bed rest for the first 12-24 hours. Supplemental oxygen may be administered if oxygen saturation is less than 90%.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
Liberal use of oxygen is associated with increased mortality in patients with acute coronary syndrome.[109]Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018 Apr 28;391(10131):1693-705.
http://www.ncbi.nlm.nih.gov/pubmed/29726345?tool=bestpractice.com
[110]Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018 Oct 24;363:k4169. Guidelines recommend that oxygen should not be routinely administered in normoxic patients with suspected or confirmed acute coronary syndrome (ACS).[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[74]National Institute for Health and Care Excellence. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. Nov 2016 [internet publication].
https://www.nice.org.uk/guidance/cg95
Aspirin is given immediately.
Adequate analgesia with morphine is essential to relieve pain and its related sympathetic activity, which can further increase myocardial oxygen demand.
Nitroglycerin should also be given immediately, if the patient is not hypotensive, as it reduces myocardial oxygen demand and lessens ischemia, and may rarely abort MI if there is coronary spasm. However, it should not be given in doses that interfere with analgesic therapy. Sublingual dosing should be given first to all patients, while intravenous therapy is reserved for patients with hypertension or heart failure.
Hemodynamically unstable
Cardiogenic shock occurs in 5% to 10% of people presenting with STEMI, and the in-hospital mortality is ≥50%.[89]Zeymer U, Bueno H, Granger CB, et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020 Mar;9(2):183-97.
https://academic.oup.com/ehjacc/article/9/2/183/5933392
http://www.ncbi.nlm.nih.gov/pubmed/32114774?tool=bestpractice.com
[90]Samsky MD, Morrow DA, Proudfoot AG, et al. Cardiogenic shock after acute myocardial infarction: a review. JAMA. 2021 Nov 9;326(18):1840-50.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9661446
http://www.ncbi.nlm.nih.gov/pubmed/34751704?tool=bestpractice.com
If revascularization with PCI fails or is not feasible, urgent coronary artery bypass graft (CABG) is recommended for patients with cardiogenic shock or hemodynamic instability.[89]Zeymer U, Bueno H, Granger CB, et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020 Mar;9(2):183-97.
https://academic.oup.com/ehjacc/article/9/2/183/5933392
http://www.ncbi.nlm.nih.gov/pubmed/32114774?tool=bestpractice.com
[90]Samsky MD, Morrow DA, Proudfoot AG, et al. Cardiogenic shock after acute myocardial infarction: a review. JAMA. 2021 Nov 9;326(18):1840-50.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9661446
http://www.ncbi.nlm.nih.gov/pubmed/34751704?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
Patients with low cardiac output states and cardiogenic shock may benefit from a dobutamine infusion.[89]Zeymer U, Bueno H, Granger CB, et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020 Mar;9(2):183-97.
https://academic.oup.com/ehjacc/article/9/2/183/5933392
http://www.ncbi.nlm.nih.gov/pubmed/32114774?tool=bestpractice.com
[90]Samsky MD, Morrow DA, Proudfoot AG, et al. Cardiogenic shock after acute myocardial infarction: a review. JAMA. 2021 Nov 9;326(18):1840-50.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9661446
http://www.ncbi.nlm.nih.gov/pubmed/34751704?tool=bestpractice.com
Guidelines state that adjunctive use of an intra-aortic balloon pump (IABP) or a ventricular mechanical circulatory support device may be reasonable in selected patients at risk of hemodynamic compromise during PCI (e.g., in patients with severe peripheral artery or aortic disease).[89]Zeymer U, Bueno H, Granger CB, et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020 Mar;9(2):183-97.
https://academic.oup.com/ehjacc/article/9/2/183/5933392
http://www.ncbi.nlm.nih.gov/pubmed/32114774?tool=bestpractice.com
[90]Samsky MD, Morrow DA, Proudfoot AG, et al. Cardiogenic shock after acute myocardial infarction: a review. JAMA. 2021 Nov 9;326(18):1840-50.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9661446
http://www.ncbi.nlm.nih.gov/pubmed/34751704?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
Results from observational studies appear to be conflicting for IABP in acute MI, and in randomized controlled trials (RCTs) it has not been shown to reduce mortality after acute MI even in patients with cardiogenic shock.[111]Ahmad Y, Sen S, Shun-Shin MJ, et al. Intra-aortic balloon pump therapy for acute myocardial infarction: a meta-analysis. JAMA Intern Med. 2015 Jun;175(6):931-9.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2210888
http://www.ncbi.nlm.nih.gov/pubmed/25822657?tool=bestpractice.com
[  ]
In people with myocardial infarction complicated by cardiogenic shock, what are the effects of intra-aortic balloon pump counterpulsation (IABP)?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1071/fullShow me the answer
]
In people with myocardial infarction complicated by cardiogenic shock, what are the effects of intra-aortic balloon pump counterpulsation (IABP)?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1071/fullShow me the answer
Antiplatelet and anticoagulant therapy
Aspirin should be given to all patients, along with ticagrelor or prasugrel.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[112]Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009 Feb 28;373(9665):723-31.
http://www.ncbi.nlm.nih.gov/pubmed/19249633?tool=bestpractice.com
Prasugrel and ticagrelor are associated with reduced ischemic events compared to clopidogrel, though there is also an increased bleeding risk with these agents.[113]Navarese EP, Khan SU, Kołodziejczak M, et al. Comparative efficacy and safety of oral P2Y(12) inhibitors in acute coronary syndrome: network meta-analysis of 52816 patients from 12 randomized trials. Circulation. 2020 Jul 14;142(2):150-60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7489363
http://www.ncbi.nlm.nih.gov/pubmed/32468837?tool=bestpractice.com
[114]Turgeon RD, Koshman SL, Youngson E, et al. Association of ticagrelor vs clopidogrel with major adverse coronary events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA Intern Med. 2020 Mar 1;180(3):420-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6990835
http://www.ncbi.nlm.nih.gov/pubmed/31930361?tool=bestpractice.com
[115]Ruiz-Nodar JM, Esteve-Pastor MA, Rivera-Caravaca JM, et al. One-year efficacy and safety of prasugrel and ticagrelor in patients with acute coronary syndromes: Results from a prospective and multicentre achilles registry. Br J Clin Pharmacol. 2020 Jun;86(6):1052-61.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7256120
http://www.ncbi.nlm.nih.gov/pubmed/31912949?tool=bestpractice.com
Prasugrel is contraindicated in patients with a history of ischemic stroke or transient ischemic attack, and its use is not recommended in patients >75 years of age or in patients with low body weight (<60 kg) due to increased bleeding risk (though dose reductions may mitigate this risk); therefore, ticagrelor is often more widely used.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[116]Goodwin MM, Desilets AR, Willett KC. Thienopyridines in acute coronary syndrome. Ann Pharmacother. 2011 Feb;45(2):207-17.
http://www.ncbi.nlm.nih.gov/pubmed/21304037?tool=bestpractice.com
[117]Menichelli M, Neumann FJ, Ndrepepa G, et al. Age- and weight-adapted dose of prasugrel versus standard dose of ticagrelor in patients with acute coronary syndromes : results from a randomized trial. Ann Intern Med. 2020 Sep 15;173(6):436-44.
http://www.ncbi.nlm.nih.gov/pubmed/32687741?tool=bestpractice.com
Ticagrelor may be associated with higher risk of bleeding and death than clopidogrel in older patients.[118]Szummer K, Montez-Rath ME, Alfredsson J, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. 2020 Nov 3;142(18):1700-8.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.050645
http://www.ncbi.nlm.nih.gov/pubmed/32867508?tool=bestpractice.com
Clopidogrel is an alternative P2Y12 inhibitor that may be used when ticagrelor and prasugrel are contraindicated or unavailable.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[118]Szummer K, Montez-Rath ME, Alfredsson J, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. 2020 Nov 3;142(18):1700-8.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.050645
http://www.ncbi.nlm.nih.gov/pubmed/32867508?tool=bestpractice.com
Cangrelor, an intravenous P2Y12 inhibitor, can be used as an adjunct to PCI to reduce the risk of periprocedural MI, repeat coronary revascularization, and stent thrombosis in patients who have not previously been treated with a P2Y12 inhibitor and are not being treated with a glycoprotein IIb/IIIa inhibitor.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[119]Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013 Dec 14;382(9909):1981-92.
http://www.ncbi.nlm.nih.gov/pubmed/24011551?tool=bestpractice.com
Unfractionated heparin is the preferred anticoagulant to be used as a single agent in addition to antiplatelet therapy. Alternatively, bivalirudin and enoxaparin can be used.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
Additional glycoprotein IIb/IIIa inhibitors (GPIIb/IIIa inhibitors) are only recommended if there is evidence of slow flow, no-reflow, or a thrombotic complication at PCI.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
Supportive measures
Adequate analgesia with morphine is essential to relieve pain and its related sympathetic activity, which can further increase myocardial oxygen demand.
Supplemental oxygen may be administered if oxygen saturation is less than 90%.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
Guidelines recommend that oxygen should not be routinely administered in normoxic patients with suspected or confirmed ACS.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[74]National Institute for Health and Care Excellence. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. Nov 2016 [internet publication].
https://www.nice.org.uk/guidance/cg95
Glycemic control, including the use of insulin where appropriate, should also be maintained, although rigid control has not been shown to be beneficial in critically ill patients.[22]American Diabetes Association. Standards of care in diabetes - 2024. Dec 2023 [internet publication].
https://diabetesjournals.org/care/issue/47/Supplement_1
Electrically unstable: post-cardiac arrest
Emergency revascularization is recommended in patients with a cardiac arrest who have been resuscitated and are hemodynamically stable and show ECG evidence for a STEMI. Staged PCI of a significant non-infarct artery stenosis is recommended after successful primary PCI in selected hemodynamically stable patients with multivessel disease.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
Alternatively, multivessel PCI may be considered at the time of primary PCI in selected patients, although the evidence supporting this strategy is weaker.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
However, physicians should consider clinical data, lesion severity/complexity, and risk of contrast nephropathy to determine the optimal PCI strategy (primary or staged).
Emergency CABG is contraindicated in post-cardiac arrest patients who are comatose.
Thrombolysis is a potential option if PCI is not readily available, but prolonged CPR is a contraindication to the use of thrombolytics.
Hypothermia is recommended for cardiac arrest patients who have been resuscitated and remain comatose.
Hemodynamically stable: PCI available within 90 minutes of first medical contact
PCI
Primary PCI, with stent placement (using bare metal stents or drug-eluting stents), is the preferred method of revascularization, provided it can be performed in a timely manner with an experienced team of operators. Primary PCI is recommended for patients presenting within 12 hours of symptom onset and can be beneficial for patients presenting between 12 and 24 hours of symptom onset, though is most effective when symptom-to-balloon times are minimized.[87]Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165.
https://academic.oup.com/eurheartj/article/40/2/87/5079120
http://www.ncbi.nlm.nih.gov/pubmed/30165437?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[107]Redfors B, Mohebi R, Giustino G, et al. Time delay, infarct size, and microvascular obstruction after primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2021 Feb;14(2):e009879.
https://www.ahajournals.org/doi/10.1161/CIRCINTERVENTIONS.120.009879
http://www.ncbi.nlm.nih.gov/pubmed/33440999?tool=bestpractice.com
[120]De Luca G, Cassetti E, Marino P. Percutaneous coronary intervention-related time delay, patient's risk profile, and survival benefits of primary angioplasty vs lytic therapy in ST-segment elevation myocardial infarction. Am J Emerg Med. 2009 Jul;27(6):712-9.
http://www.ncbi.nlm.nih.gov/pubmed/19751630?tool=bestpractice.com
[121]Nielsen PH, Maeng M, Busk M, et al; DANAMI-2 Investigators. Primary angioplasty versus fibrinolysis in acute myocardial infarction: long-term follow-up in the Danish acute myocardial infarction 2 trial. Circulation. 2010 Apr 6;121(13):1484-91.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.109.873224
http://www.ncbi.nlm.nih.gov/pubmed/20308618?tool=bestpractice.com
It involves immediate transfer to the catheterization laboratory with the intention of opening the artery with stent placement. Drug-eluting stents are preferred.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[122]Wallace EL, Abdel-Latif A, Charnigo R, et al. Meta-analysis of long-term outcomes for drug-eluting stents versus bare-metal stents in primary percutaneous coronary interventions for ST-segment elevation myocardial infarction. Am J Cardiol. 2012 Apr 1;109(7):932-40.
http://www.ncbi.nlm.nih.gov/pubmed/22221949?tool=bestpractice.com
[123]Brugaletta S, Gomez-Lara J, Ortega-Paz L, et al. 10-Year follow-up of patients with everolimus-eluting versus bare-metal stents after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021 Mar 9;77(9):1165-78.
https://www.sciencedirect.com/science/article/pii/S073510972100019X
http://www.ncbi.nlm.nih.gov/pubmed/33663733?tool=bestpractice.com
[  ]
How do drug-eluting stents compare with bare-metal stents for people with acute coronary syndrome?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.1890/fullShow me the answer Third-generation drug-eluting stents composed of biodegradable polymers are currently being investigated.[124]Raungaard B, Jensen LO, Tilsted HH, et al; Scandinavian Organization for Randomized Trials with Clinical Outcome (SORT OUT). Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised non-inferiority trial. Lancet. 2015 Apr 18;385(9977):1527-35.
http://www.ncbi.nlm.nih.gov/pubmed/25601789?tool=bestpractice.com
[125]Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016 Jan 23;387(10016):357-66.
https://boris.unibe.ch/92887
http://www.ncbi.nlm.nih.gov/pubmed/26520230?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Angiogram showing occluded right coronary arteryFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].
]
How do drug-eluting stents compare with bare-metal stents for people with acute coronary syndrome?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.1890/fullShow me the answer Third-generation drug-eluting stents composed of biodegradable polymers are currently being investigated.[124]Raungaard B, Jensen LO, Tilsted HH, et al; Scandinavian Organization for Randomized Trials with Clinical Outcome (SORT OUT). Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised non-inferiority trial. Lancet. 2015 Apr 18;385(9977):1527-35.
http://www.ncbi.nlm.nih.gov/pubmed/25601789?tool=bestpractice.com
[125]Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016 Jan 23;387(10016):357-66.
https://boris.unibe.ch/92887
http://www.ncbi.nlm.nih.gov/pubmed/26520230?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Angiogram showing occluded right coronary arteryFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].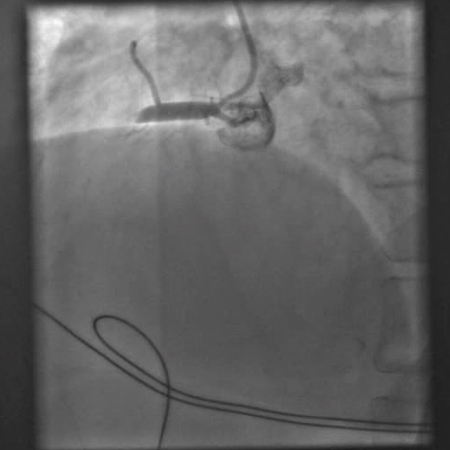 [Figure caption and citation for the preceding image starts]: Angiogram showing an attempt to open the occluded right coronary artery with an angioplasty balloonFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Angiogram showing an attempt to open the occluded right coronary artery with an angioplasty balloonFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Angiogram after balloon angioplasty and stenting showing an open right coronary arteryFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Angiogram after balloon angioplasty and stenting showing an open right coronary arteryFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].
Radial approach is preferable to femoral approach as it results in better outcomes (e.g., reduction in mortality, major adverse cardiovascular events, major bleeding, and bleeding complications), particularly if the operator is experienced in radial access.[87]Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165.
https://academic.oup.com/eurheartj/article/40/2/87/5079120
http://www.ncbi.nlm.nih.gov/pubmed/30165437?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[126]Andò G, Capodanno D. Radial versus femoral access in invasively managed patients with acute coronary syndrome: a systematic review and meta-analysis. Ann Intern Med. 2015 Dec 15;163(12):932-40.
http://www.ncbi.nlm.nih.gov/pubmed/26551857?tool=bestpractice.com
[127]Valgimigli M, Gagnor A, Calabró P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015 Jun 20;385(9986):2465-76.
http://www.ncbi.nlm.nih.gov/pubmed/25791214?tool=bestpractice.com
[128]Nardin M, Verdoia M, Barbieri L, et al. Radial vs femoral approach in acute coronary syndromes: a meta-analysis of randomized trials. Curr Vasc Pharmacol. 2017;16(1):79-92.
http://www.ncbi.nlm.nih.gov/pubmed/28490313?tool=bestpractice.com
Staged PCI of a significant non-infarct artery stenosis is recommended after successful primary PCI in selected hemodynamically stable patients with STEMI and multivessel disease.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
Alternatively, multivessel PCI may be considered at the time of primary PCI in selected patients, although the evidence supporting this strategy is weaker.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[129]Engstrøm T, Kelbæk H, Helqvist S, et al; DANAMI-3-PRIMULTI Investigators. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015 Aug 15;386(9994):665-71.
http://www.ncbi.nlm.nih.gov/pubmed/26347918?tool=bestpractice.com
[130]Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015 Mar 17;65(10):963-72.
https://www.jacc.org/doi/10.1016/j.jacc.2014.12.038
http://www.ncbi.nlm.nih.gov/pubmed/25766941?tool=bestpractice.com
[131]Kowalewski M, Schulze V, Berti S, et al. Complete revascularisation in ST-elevation myocardial infarction and multivessel disease: meta-analysis of randomised controlled trials. Heart. 2015 Aug;101(16):1309-17.
http://www.ncbi.nlm.nih.gov/pubmed/26037102?tool=bestpractice.com
Physicians should consider factors such as clinical data, hemodynamic stability, lesion severity/complexity, and risk of contrast nephropathy to determine the optimal PCI strategy. Calculation of the SYNTAX score is recommended for left main or multivessel PCI.
SYNTAX score
Opens in new window
Guidelines also recommend against routine manual aspiration thrombectomy before primary PCI as the evidence does not suggest any benefit of this procedure over PCI alone.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[132]Bhindi R, Kajander OA, Jolly SS, et al. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention-alone in ST-segment elevation myocardial infarction: the optical coherence tomography sub-study of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Eur Heart J. 2015 Aug 1;36(29):1892-900.
https://academic.oup.com/eurheartj/article/36/29/1892/2465937
http://www.ncbi.nlm.nih.gov/pubmed/25994742?tool=bestpractice.com
Furthermore, studies have suggested that routine aspiration thrombectomy might increase the risk of stroke.[133]Jolly SS, Cairns JA, Yusuf S, et al; TOTAL Investigators. Stroke in the TOTAL trial: a randomized trial of routine thrombectomy vs. percutaneous coronary intervention alone in ST elevation myocardial infarction. Eur Heart J. 2015 Sep 14;36(35):2364-72.
https://academic.oup.com/eurheartj/article/36/35/2364/2466054
http://www.ncbi.nlm.nih.gov/pubmed/26129947?tool=bestpractice.com
[134]Jolly SS, Cairns JA, Yusuf S, et al; TOTAL Investigators. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016 Jan 9;387(10014):127-35.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5007127
http://www.ncbi.nlm.nih.gov/pubmed/26474811?tool=bestpractice.com
Many hospitals have 24-hour PCI capacity; however, in facilities without catheterization laboratories, routine transfer to a PCI facility should be considered for all patients if transfer times are reasonable and total ischemic time after presentation is less than 120 minutes.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
CABG
Emergency revascularization with CABG can be useful if PCI fails or is not feasible, and a large area of myocardium is at risk.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
CABG is recommended for patients with cardiogenic shock or heart failure if PCI is not feasible.[89]Zeymer U, Bueno H, Granger CB, et al. Acute Cardiovascular Care Association position statement for the diagnosis and treatment of patients with acute myocardial infarction complicated by cardiogenic shock: a document of the Acute Cardiovascular Care Association of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2020 Mar;9(2):183-97.
https://academic.oup.com/ehjacc/article/9/2/183/5933392
http://www.ncbi.nlm.nih.gov/pubmed/32114774?tool=bestpractice.com
[90]Samsky MD, Morrow DA, Proudfoot AG, et al. Cardiogenic shock after acute myocardial infarction: a review. JAMA. 2021 Nov 9;326(18):1840-50.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9661446
http://www.ncbi.nlm.nih.gov/pubmed/34751704?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
CABG should not be undertaken after failed primary PCI in the absence of ischemia or a large area of myocardium at risk, or if surgical revascularization is not feasible due to a no-reflow state or poor distal targets.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
Antiplatelet and anticoagulant therapy
Aspirin should be given to all patients, along with ticagrelor or prasugrel.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[112]Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009 Feb 28;373(9665):723-31.
http://www.ncbi.nlm.nih.gov/pubmed/19249633?tool=bestpractice.com
Prasugrel and ticagrelor are associated with reduced ischemic events compared to clopidogrel, though there is also an increased bleeding risk with these agents.[113]Navarese EP, Khan SU, Kołodziejczak M, et al. Comparative efficacy and safety of oral P2Y(12) inhibitors in acute coronary syndrome: network meta-analysis of 52816 patients from 12 randomized trials. Circulation. 2020 Jul 14;142(2):150-60.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7489363
http://www.ncbi.nlm.nih.gov/pubmed/32468837?tool=bestpractice.com
[114]Turgeon RD, Koshman SL, Youngson E, et al. Association of ticagrelor vs clopidogrel with major adverse coronary events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA Intern Med. 2020 Mar 1;180(3):420-8.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6990835
http://www.ncbi.nlm.nih.gov/pubmed/31930361?tool=bestpractice.com
[115]Ruiz-Nodar JM, Esteve-Pastor MA, Rivera-Caravaca JM, et al. One-year efficacy and safety of prasugrel and ticagrelor in patients with acute coronary syndromes: Results from a prospective and multicentre achilles registry. Br J Clin Pharmacol. 2020 Jun;86(6):1052-61.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7256120
http://www.ncbi.nlm.nih.gov/pubmed/31912949?tool=bestpractice.com
Prasugrel is contraindicated in patients with a history of ischemic stroke or transient ischemic attack, and its use is not recommended in patients >75 years of age or in patients with low body weight (<60 kg) due to increased bleeding risk (though dose reductions may mitigate this risk); therefore, ticagrelor is often more widely used.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[116]Goodwin MM, Desilets AR, Willett KC. Thienopyridines in acute coronary syndrome. Ann Pharmacother. 2011 Feb;45(2):207-17.
http://www.ncbi.nlm.nih.gov/pubmed/21304037?tool=bestpractice.com
[117]Menichelli M, Neumann FJ, Ndrepepa G, et al. Age- and weight-adapted dose of prasugrel versus standard dose of ticagrelor in patients with acute coronary syndromes : results from a randomized trial. Ann Intern Med. 2020 Sep 15;173(6):436-44.
http://www.ncbi.nlm.nih.gov/pubmed/32687741?tool=bestpractice.com
Ticagrelor may be associated with higher risk of bleeding and death than clopidogrel in older patients.[118]Szummer K, Montez-Rath ME, Alfredsson J, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. 2020 Nov 3;142(18):1700-8.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.050645
http://www.ncbi.nlm.nih.gov/pubmed/32867508?tool=bestpractice.com
Clopidogrel is an alternative P2Y12 inhibitor that may be used when ticagrelor and prasugrel are contraindicated or unavailable.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[118]Szummer K, Montez-Rath ME, Alfredsson J, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. 2020 Nov 3;142(18):1700-8.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.050645
http://www.ncbi.nlm.nih.gov/pubmed/32867508?tool=bestpractice.com
Cangrelor, an intravenous P2Y12 inhibitor, can be used as an adjunct to PCI to reduce the risk of periprocedural MI, repeat coronary revascularization, and stent thrombosis in patients who have not previously been treated with a P2Y12 inhibitor and are not being treated with a GPIIb/IIIa inhibitor.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[119]Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet. 2013 Dec 14;382(9909):1981-92.
http://www.ncbi.nlm.nih.gov/pubmed/24011551?tool=bestpractice.com
Unfractionated heparin is the preferred anticoagulant to be used as a single agent in addition to antiplatelet therapy. Alternatively, bivalirudin and enoxaparin can be used. Additional glycoprotein IIb/IIIa inhibitors (GPIIb/IIIa inhibitors) are only recommended if there is evidence of slow flow, no-flow, or a thrombotic complication at PCI.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
Supportive measures
Oral beta-blockers should be started as soon as possible, as they decrease infarction size and reduce mortality, although care should be taken in patients with evidence of heart failure, hypotension, bradycardia, or asthma.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[135]Bangalore S, Makani H, Radford M, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. 2014 Oct;127(10):939-53.
https://www.amjmed.com/article/S0002-9343(14)00470-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/24927909?tool=bestpractice.com
[136]Peck KY, Andrianopoulos N, Dinh D, et al. Role of beta blockers following percutaneous coronary intervention for acute coronary syndrome. Heart. 2021 May;107(9):728-33.
http://www.ncbi.nlm.nih.gov/pubmed/32887736?tool=bestpractice.com
Intravenous beta-blockers are recommended only in patients who are hypertensive or have ongoing ischemia, provided there are no contraindications to their use.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[137]Pizarro G, Fernández-Friera L, Fuster V, et al. Long-term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction). J Am Coll Cardiol. 2014 Jun 10;63(22):2356-62.
https://www.jacc.org/doi/10.1016/j.jacc.2014.03.014
http://www.ncbi.nlm.nih.gov/pubmed/24694530?tool=bestpractice.com
In the absence of contraindications, high-intensity statin therapy (i.e., statin regimens that reduce LDL-cholesterol by ≥50%) should be initiated or continued in all stabilized patients with STEMI.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[138]Schubert J, Lindahl B, Melhus H, et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J. 2021 Jan 20;42(3):243-52.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7954251
http://www.ncbi.nlm.nih.gov/pubmed/33367526?tool=bestpractice.com
Eplerenone should be added to optimal medical therapy in eligible patients (creatinine <2.5 mg/dL in men and <2.0 mg/dL in women; potassium <5.0 mEq/L) 3-14 days after STEMI with ejection fraction <0.40, and either symptomatic heart failure or diabetes mellitus.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[139]Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309-21.
https://www.nejm.org/doi/10.1056/NEJMoa030207
http://www.ncbi.nlm.nih.gov/pubmed/12668699?tool=bestpractice.com
Earlier initiation of the drug (<7 days) has been shown to significantly reduce the rates of all-cause mortality, sudden cardiac death, and cardiovascular mortality/hospitalization, whereas initiation >7 days has not been shown to have a significant effect on outcomes.[140]Montalescot G, Pitt B, Lopez de Sa E, et al; REMINDER Investigators. Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: the Randomized Double-Blind Reminder Study. Eur Heart J. 2014 Sep 7;35(34):2295-302.
https://academic.oup.com/eurheartj/article/35/34/2295/2481156
http://www.ncbi.nlm.nih.gov/pubmed/24780614?tool=bestpractice.com
Supplemental oxygen may be administered if oxygen saturation is less than 90%.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
Guidelines recommend that oxygen should not be routinely administered in normoxic patients with suspected or confirmed ACS.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[74]National Institute for Health and Care Excellence. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. Nov 2016 [internet publication].
https://www.nice.org.uk/guidance/cg95
Glycemic control, including the use of insulin where appropriate, should also be maintained, although rigid control has not been shown to be beneficial in critically ill patients.[22]American Diabetes Association. Standards of care in diabetes - 2024. Dec 2023 [internet publication].
https://diabetesjournals.org/care/issue/47/Supplement_1
Nonsteroidal anti-inflammatory drugs should be avoided, and stopped if possible in patients already on them.[141]Gibson CM, Pride YB, Aylward PE, et al. Association of non-steroidal anti-inflammatory drugs with outcomes in patients with ST-segment elevation myocardial infarction treated with fibrinolytic therapy: an ExTRACT-TIMI 25 analysis. J Thromb Thrombolysis. 2009 Jan;27(1):11-7.
http://www.ncbi.nlm.nih.gov/pubmed/18695943?tool=bestpractice.com
Hemodynamically stable: PCI available >90 minutes after first medical contact and within 12 hours of symptom onset with no contraindications to thrombolysis
Thrombolysis is indicated if PCI is not available within 90 minutes of first medical contact and the patient has no contraindications to thrombolytic therapy.[142]Armstrong PW, Gershlick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013 Apr 11;368(15):1379-87.
https://www.nejm.org/doi/10.1056/NEJMoa1301092
http://www.ncbi.nlm.nih.gov/pubmed/23473396?tool=bestpractice.com
[143]Jamal J, Idris H, Faour A, et al. Late outcomes of ST-elevation myocardial infarction treated by pharmaco-invasive or primary percutaneous coronary intervention. Eur Heart J. 2023 Feb 7;44(6):516-28.
https://academic.oup.com/eurheartj/article/44/6/516/6865195
http://www.ncbi.nlm.nih.gov/pubmed/36459120?tool=bestpractice.com
It should be initiated within 30 minutes of presentation. Thrombolysis is used only once on initial diagnosis, and this must be within 12 hours of symptom onset (ideally within 3 hours) as the efficacy of fibrinolytic agents in lysing the thrombus diminishes over time. Therapy within the first 2 hours (particularly the first hour) can occasionally abort MI and dramatically reduce mortality.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
Absolute contraindications for thrombolysis are any prior intracranial hemorrhage, known malignant intracranial lesion or structural cerebral vascular lesion (e.g., arteriovenous malformations), ischemic stroke within previous 3 months, suspected aortic dissection, active bleeding or bleeding diathesis, and significant closed head or facial trauma within previous 3 months.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
Thrombolytics can be associated with an increased risk of bleeding, in addition to the risk associated with other antithrombotic and/or antiplatelet agents used, and can also cause intracranial hemorrhage.
Transfer to a PCI-capable hospital within 24 hours should be considered in all patients undergoing fibrinolytic therapy. Angiography with intent to fully revascularize the culprit vessel should be considered within 24 hours even after successful fibrinolytic therapy (i.e., treatment of residual stenosis or suboptimal flow of the infarct artery).[87]Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019 Jan 7;40(2):87-165.
https://academic.oup.com/eurheartj/article/40/2/87/5079120
http://www.ncbi.nlm.nih.gov/pubmed/30165437?tool=bestpractice.com
[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[143]Jamal J, Idris H, Faour A, et al. Late outcomes of ST-elevation myocardial infarction treated by pharmaco-invasive or primary percutaneous coronary intervention. Eur Heart J. 2023 Feb 7;44(6):516-28.
https://academic.oup.com/eurheartj/article/44/6/516/6865195
http://www.ncbi.nlm.nih.gov/pubmed/36459120?tool=bestpractice.com
Rescue PCI after thrombolysis is recommended in patients with evidence of failed reperfusion (such as ongoing chest pain; hemodynamic, mechanical, or electrical instability; or shock). Treated patients should be transferred for PCI as soon as possible after thrombolysis.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[144]Borgia F, Goodman SG, Halvorsen S, et al. Early routine percutaneous coronary intervention after fibrinolysis vs. standard therapy in ST-segment elevation myocardial infarction: a meta-analysis. Eur Heart J. 2010 Sep;31(17):2156-69.
https://academic.oup.com/eurheartj/article/31/17/2156/464143
http://www.ncbi.nlm.nih.gov/pubmed/20601393?tool=bestpractice.com
Those transferred within 6 hours after thrombolytic therapy had significantly fewer ischemic complications than those who were only transferred if they had complications.[145]Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol. 2007 Jan 30;49(4):422-30.
https://www.jacc.org/doi/10.1016/j.jacc.2006.09.033
http://www.ncbi.nlm.nih.gov/pubmed/17258087?tool=bestpractice.com
Rescue PCI is associated with improved clinical outcomes after failed fibrinolytic therapy.[146]Cantor WJ, Fitchett D, Borgundvaag B, et al. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med. 2009 Jun 25;360(26):2705-18.
https://www.nejm.org/doi/10.1056/NEJMoa0808276
http://www.ncbi.nlm.nih.gov/pubmed/19553646?tool=bestpractice.com
Antiplatelet and anticoagulant agents (e.g., oral aspirin and clopidogrel; intravenous heparin) are also indicated for the treatment of STEMI, as this limits secondary thrombosis by inhibiting platelet activation and subsequent platelet aggregation. Prasugrel and ticagrelor are not recommended in patients undergoing thrombolysis as they have not been adequately studied in this setting.[147]Beygui F, Castren M, Brunetti ND, et al. Pre-hospital management of patients with chest pain and/or dyspnoea of cardiac origin. A position paper of the Acute Cardiovascular Care Association (ACCA) of the ESC. Eur Heart J Acute Cardiovasc Care. 2020 Mar;9(1):59-81.
https://academic.oup.com/ehjacc/article/9/1_suppl/59/5923956
http://www.ncbi.nlm.nih.gov/pubmed/26315695?tool=bestpractice.com
GPIIb/IIIa inhibitors are not indicated in STEMI if thrombolytic therapy is indicated. Low molecular weight heparin should be considered instead of unfractionated heparin in patients treated with thrombolysis.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[148]Silvain J, Beygui F, Barthélémy O, et al. Efficacy and safety of enoxaparin versus unfractionated heparin during percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2012 Feb 3;344:e553.
https://www.bmj.com/content/344/bmj.e553
http://www.ncbi.nlm.nih.gov/pubmed/22306479?tool=bestpractice.com
[149]Singh S, Bahekar A, Molnar J, et al. Adjunctive low molecular weight heparin during fibrinolytic therapy in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Clin Cardiol. 2009 Jul;32(7):358-64.
http://www.ncbi.nlm.nih.gov/pubmed/19609890?tool=bestpractice.com
Supportive measures are the same as those for patients who undergo PCI within 90 minutes.
Hemodynamically stable: PCI available >90 minutes after first medical contact and within 12 hours of symptom onset with contraindications to thrombolysis
In patients with contraindications to thrombolysis, PCI is indicated even if it cannot occur within 90 minutes. Patients should be transferred for PCI as soon as possible.
Antiplatelet/anticoagulant agents and supportive measures are the same as those for patients who undergo PCI within 90 minutes.
Hemodynamically stable: no access to PCI within 90 minutes and >12 hours after symptom onset
Even beyond 12 hours, if there are persistent symptoms, it is possible to obtain benefits from revascularization, which would be best performed with percutaneous coronary revascularization. Stable patients in whom PCI or thrombolysis is not indicated are treated with pharmacotherapy only, including beta-blockers and antiplatelet and anticoagulation therapy. If the patient becomes unstable, they should undergo late PCI, which can be done up to 36 hours after the onset of symptoms.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[150]Hochman JS, Sleeper LA, Godfrey E, et al. Should we emergently revascularize occluded coronaries for cardiogenic shock: an international randomized trial of emergency PTCA/CABG-trial design. The SHOCK Trial Study Group. Am Heart J. 1999 Feb;137(2):313-21.
https://www.sciencedirect.com/science/article/pii/S0002870399005074
http://www.ncbi.nlm.nih.gov/pubmed/9924166?tool=bestpractice.com
Routine primary PCI strategy should still be considered in patients presenting between 12 and 48 hours after symptom onset. However, if the time since symptom onset is >48 hours and the patient is now asymptomatic, routine PCI of an occluded infarct-related artery is not recommended.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[151]British Cardiovascular Society. PCI in late-presenting STEMI: how late is too late? Jan 2022 [internet publication].
https://www.britishcardiovascularsociety.org/resources/editorials/articles/pci-late-presenting-stemi
Supportive measures are the same as those for patients who undergo PCI within 90 minutes.
Post-STEMI
The American College of Cardiology/American Heart Association guidelines recommend that, where available, cardiac rehabilitation/secondary prevention programs are provided for patients with STEMI, particularly those with multiple modifiable risk factors and/or those moderate- to high-risk patients in whom supervised exercise training is warranted.[152]Thomas RJ, Beatty AL, Beckie TM, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019 Jul 2;140(1):e69-89.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000663
http://www.ncbi.nlm.nih.gov/pubmed/31082266?tool=bestpractice.com
[153]Smith SC Jr, Benjamin EJ, Bonow RO, et al; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update. 2011 Nov 29;124(22):2458-73.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e318235eb4d
[154]Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women - 2011 update: a guideline from the American Heart Association. Circulation. 2011 Mar 22;123(11):1243-62.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e31820faaf8
http://www.ncbi.nlm.nih.gov/pubmed/21325087?tool=bestpractice.com
[  ]
What are the effects of exercise‐based cardiac rehabilitation for people with coronary heart disease?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3897/fullShow me the answer[Evidence A]b44c575a-3fe8-4a4e-bdc4-85757b30e402ccaAWhat are the effects of exercise‐based cardiac rehabilitation for people with coronary heart disease? Systematic review evidence has shown exercise‐based cardiac rehabilitation helps to improve outcomes in people with coronary heart disease.[155]Dibben G, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021 Nov 6;11(11):CD001800.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001800.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/34741536?tool=bestpractice.com
]
What are the effects of exercise‐based cardiac rehabilitation for people with coronary heart disease?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3897/fullShow me the answer[Evidence A]b44c575a-3fe8-4a4e-bdc4-85757b30e402ccaAWhat are the effects of exercise‐based cardiac rehabilitation for people with coronary heart disease? Systematic review evidence has shown exercise‐based cardiac rehabilitation helps to improve outcomes in people with coronary heart disease.[155]Dibben G, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021 Nov 6;11(11):CD001800.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001800.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/34741536?tool=bestpractice.com
Patients without any preexisting risk factors for cardiovascular disease (CVD) are at increased risk of early mortality; even patients who are deemed low risk require prompt initiation of evidence-based pharmacotherapy post ACS.[156]Figtree GA, Vernon ST, Hadziosmanovic N, et al. Mortality in STEMI patients without standard modifiable risk factors: a sex-disaggregated analysis of SWEDEHEART registry data. Lancet. 2021 Mar 20;397(10279):1085-94.
http://www.ncbi.nlm.nih.gov/pubmed/33711294?tool=bestpractice.com
Dual antiplatelet therapy should usually be continued in all patients for at least 12 months, whether they were stented or not.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[157]Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018 Jan 14;39(3):213-60.
https://academic.oup.com/eurheartj/article/39/3/213/4095043
http://www.ncbi.nlm.nih.gov/pubmed/28886622?tool=bestpractice.com
In selected patients undergoing PCI, shorter-duration dual antiplatelet therapy (1-3 months) can be considered, with subsequent transition to P2Y12 inhibitor monotherapy to reduce the risk of bleeding events.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
[158]Kim BK, Hong SJ, Cho YH, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020 Jun 16;323(23):2407-16.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7298605
http://www.ncbi.nlm.nih.gov/pubmed/32543684?tool=bestpractice.com
[159]Kim HS, Kang J, Hwang D, et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): an open-label, multicentre, non-inferiority randomised trial. Lancet. 2020 Oct 10;396(10257):1079-89.
http://www.ncbi.nlm.nih.gov/pubmed/32882163?tool=bestpractice.com
[160]O'Donoghue ML, Murphy SA, Sabatine MS. The safety and efficacy of aspirin discontinuation on a background of a P2Y(12) inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation. 2020 Aug 11;142(6):538-45.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.046251
http://www.ncbi.nlm.nih.gov/pubmed/32551860?tool=bestpractice.com
[161]Khan SU, Singh M, Valavoor S, et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation. 2020 Oct 13;142(15):1425-36.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7547897
http://www.ncbi.nlm.nih.gov/pubmed/32795096?tool=bestpractice.com
[162]Giacoppo D, Matsuda Y, Fovino LN, et al. Short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy vs. prolonged dual antiplatelet therapy after percutaneous coronary intervention with second-generation drug-eluting stents: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2021 Jan 21;42(4):308-19.
https://academic.oup.com/eurheartj/article/42/4/308/6025040
http://www.ncbi.nlm.nih.gov/pubmed/33284979?tool=bestpractice.com
Statin therapy should be given indefinitely if tolerated and not contraindicated.[69]O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Jan 29;127(4):e362-425.
https://www.ahajournals.org/doi/10.1161/CIR.0b013e3182742cf6
http://www.ncbi.nlm.nih.gov/pubmed/23247304?tool=bestpractice.com
[138]Schubert J, Lindahl B, Melhus H, et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J. 2021 Jan 20;42(3):243-52.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7954251
http://www.ncbi.nlm.nih.gov/pubmed/33367526?tool=bestpractice.com
For patients at very high risk of future events, and those up to 75 years of age and not at very high risk, ezetimibe may be added if the patient is on maximal statin therapy, and LDL-cholesterol level remains ≥70 mg/dL.[163]Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Jun 18;139(25):e1082-143.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000625
http://www.ncbi.nlm.nih.gov/pubmed/30586774?tool=bestpractice.com
A proprotein convertase subtilisin/kexin type 9 (PCSK9) antibody inhibitor (evolocumab or alirocumab) may be added to maximal statin and ezetimibe therapy if the patient is at very high risk of future events and LDL-cholesterol level remains ≥70 mg/dL, or non-HDL-cholesterol ≥100 mg/dL.[3]Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Oct 12;44(38):3720-826.
https://academic.oup.com/eurheartj/article/44/38/3720/7243210
http://www.ncbi.nlm.nih.gov/pubmed/37622654?tool=bestpractice.com
[43]Szarek M, Bittner VA, Aylward P, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY outcomes trial. Eur Heart J. 2020 Nov 21;41(44):4245-55.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7724642
http://www.ncbi.nlm.nih.gov/pubmed/33051646?tool=bestpractice.com
[163]Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Jun 18;139(25):e1082-143.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000625
http://www.ncbi.nlm.nih.gov/pubmed/30586774?tool=bestpractice.com
[164]Oyama K, Giugliano RP, Tang M, et al. Effect of evolocumab on acute arterial events across all vascular territories: results from the FOURIER trial. Eur Heart J. 2021 Dec 14;42(47):4821-9.
https://academic.oup.com/eurheartj/article/42/47/4821/6372436
http://www.ncbi.nlm.nih.gov/pubmed/34537830?tool=bestpractice.com
Patients are considered to be at very high risk of future events if they have a history of multiple major atherosclerotic CVD events (recent ACS [within the past 12 months], MI other than the recent ACS, ischemic stroke, symptomatic peripheral arterial disease [claudication with ankle brachial index <0.85, previous revascularization or amputation]), or one major atherosclerotic CVD event and multiple high risk conditions (age ≥65 years, heterozygous family history, history of previous CABG or PCI, diabetes mellitus, hypertension, chronic kidney disease, current smoking, persistently elevated LDL-cholesterol [≥100 mg/dL {≥2.6 mmol/L}] despite maximally tolerated therapy, history of congestive heart failure).[163]Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Jun 18;139(25):e1082-143.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000625
http://www.ncbi.nlm.nih.gov/pubmed/30586774?tool=bestpractice.com
ACE inhibitors should be started early (i.e., when patient is hemodynamically stable, optimally on first day in the hospital) for a favorable effect on ventricular remodeling, especially in patients with large anterior wall MI. Beta-blockers should be continued long term (>1 year) - continuing use should then be evaluated on the basis of comorbidities.[135]Bangalore S, Makani H, Radford M, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. 2014 Oct;127(10):939-53.
https://www.amjmed.com/article/S0002-9343(14)00470-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/24927909?tool=bestpractice.com
[165]Safi S, Sethi NJ, Korang SK, et al. Beta-blockers in patients without heart failure after myocardial infarction. Cochrane Database Syst Rev. 2021 Nov 5;11(11):CD012565.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8570410
http://www.ncbi.nlm.nih.gov/pubmed/34739733?tool=bestpractice.com
[166]Virani SS, Newby LK, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. Circulation. 2023 Aug 29;148(9):e9-119.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001168
http://www.ncbi.nlm.nih.gov/pubmed/37471501?tool=bestpractice.com
[167]Kim J, Kang D, Park H, et al. Long-term β-blocker therapy and clinical outcomes after acute myocardial infarction in patients without heart failure: nationwide cohort study. Eur Heart J. 2020 Oct 1;41(37):3521-9.
https://academic.oup.com/eurheartj/article/41/37/3521/5857797
http://www.ncbi.nlm.nih.gov/pubmed/32542362?tool=bestpractice.com
The American College of Cardiology/American Heart Association recommend that the decision to continue beta-blockers long term after revascularization should be made on an individualized basis.[106]Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 Jan 18;145(3):e18-114.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
A sodium-glucose cotransporter-2 (SGLT2) inhibitor, such as dapagliflozin or empagliflozin, should be given to patients with heart failure when they are clinically stable, regardless of their left ventricular ejection fraction.[168]McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-726.
https://academic.oup.com/eurheartj/article/42/36/3599/6358045
[169]McDonagh TA, Metra M, Adamo M, et al. 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023 Oct 1;44(37):3627-39.
https://academic.oup.com/eurheartj/article/44/37/3627/7246292
[170]von Lewinski D, Kolesnik E, Tripolt NJ, et al. Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J. 2022 Nov 1;43(41):4421-32.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9622301
http://www.ncbi.nlm.nih.gov/pubmed/36036746?tool=bestpractice.com
Chronic nitrate use is not routinely recommended after a MI, but may be used as part of the treatment plan for congestive heart failure and for chronic angina.
 ]
]
 ]
Third-generation drug-eluting stents composed of biodegradable polymers are currently being investigated.[124][125][Figure caption and citation for the preceding image starts]: Angiogram showing occluded right coronary arteryFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].
]
Third-generation drug-eluting stents composed of biodegradable polymers are currently being investigated.[124][125][Figure caption and citation for the preceding image starts]: Angiogram showing occluded right coronary arteryFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Angiogram showing an attempt to open the occluded right coronary artery with an angioplasty balloonFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Angiogram showing an attempt to open the occluded right coronary artery with an angioplasty balloonFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Angiogram after balloon angioplasty and stenting showing an open right coronary arteryFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Angiogram after balloon angioplasty and stenting showing an open right coronary arteryFrom the personal collection of Dr Mahi Ashwath; used with permission [Citation ends].
 ]
[Evidence A] Systematic review evidence has shown exercise‐based cardiac rehabilitation helps to improve outcomes in people with coronary heart disease.[155]
]
[Evidence A] Systematic review evidence has shown exercise‐based cardiac rehabilitation helps to improve outcomes in people with coronary heart disease.[155]