Hemodynamically unstable patients require urgent primary reperfusion, anticoagulation, and supportive care.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
For patients at intermediate risk of a poor outcome, anticoagulation and ongoing monitoring is required. Reperfusion is generally employed as a rescue therapy if decompensation occurs.
Patients at low risk of a poor outcome can be managed as outpatients, taking into account the patient’s personal circumstances, and as long as all the following criteria are met:[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Clinically stable with good cardiopulmonary reserve
No contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (i.e., <50,000/mm³)
Expected adherence to treatment
The patient feels well enough to be treated at home.
Patients who are incidentally diagnosed with asymptomatic PE should receive the same initial and long-term anticoagulation as those with comparable symptomatic PE.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Classification of severity
Several classification systems have been employed to describe the severity of PE, and short-term mortality may be assessed by clinical prediction tools such as the Pulmonary Embolism Severity Index (PESI) or simplified PESI scores.[155]Tong C, Zhang Z. Evaluation factors of pulmonary embolism severity and prognosis. Clin Appl Thromb Hemost. 2015 Apr;21(3):273-84.
https://www.doi.org/10.1177/1076029613501540
http://www.ncbi.nlm.nih.gov/pubmed/24023267?tool=bestpractice.com
The term "submassive" has been applied to PE with significant anatomic extent, but with normotension, and massive describes anatomically extensive PE complicated by shock or hypotension.
The European Society of Cardiology categorizes PE as:
High risk when presenting with hemodynamic instability (shock or hypotension)
Intermediate-high risk when presenting without hemodynamic instability but with evidence of right ventricular (RV) dysfunction on imaging, abnormal cardiac biomarkers and clinical parameters of severity (such as a high PESI score)
Intermediate-low risk when presenting without hemodynamic instability, with either evidence of RV dysfunction on imaging or elevated cardiac biomarkers (but not both) and clinical parameters of severity (such as a high PESI score)
Low risk when none of these factors are present.
Suspected PE with shock or hypotension
High-risk patients (presenting with shock or hypotension [i.e., systolic BP <90 mmHg]) require aggressive treatment with primary reperfusion, anticoagulation, and supportive therapy.
Supportive therapies and empiric anticoagulation (unless contraindicated) should be instituted without delay.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Unfractionated heparin (UFH) may be preferred in this population; most clinical studies of interventional therapies have used heparin as the anticoagulant component of the regimen.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Supportive therapies
Local resuscitation protocols should be followed.
Respiratory support
Supplemental high-concentration oxygen should be administered, targeting oxygen saturations of 94% to 98% (or 88% to 92% in patients at risk of hypercapnic respiratory failure).[156]O'Driscoll BR, Howard LS, Earis J, et al; British Thoracic Society Emergency Oxygen Guideline Group; BTS Emergency Oxygen Guideline Development Group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(Suppl 1):ii1-90.
https://www.brit-thoracic.org.uk/document-library/clinical-information/oxygen/2017-emergency-oxygen-guideline/bts-guideline-for-oxygen-use-in-adults-in-healthcare-and-emergency-settings
http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
Intubation and mechanical ventilation may be necessary for patients with severe hypoxemia/respiratory failure. Mechanical ventilation can lead to hypotension, so BP should be monitored closely.
Intravenous fluids
If systolic BP is <90 mmHg, intravenous fluids should be given. Acute right ventricular failure with resulting low systemic output is the leading cause of death in patients with PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Studies indicate that aggressive volume expansion is of no benefit, and may even worsen right ventricular function by causing mechanical overstretch, or by reflex mechanisms that depress contractility. However, modest fluid challenge (i.e., 500 mL crystalloid) may be of benefit in patients with PE, a low cardiac index, and normal BP.[160]Mercat A, Diehl JL, Meyer G, et al. Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med. 1999 Mar;27(3):540-4.
http://www.ncbi.nlm.nih.gov/pubmed/10199533?tool=bestpractice.com
Vasoactive agents
If systolic BP is <90 mmHg, vasopressors may be given in parallel with (or while waiting for) pharmacologic, surgical, or interventional reperfusion treatment.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Norepinephrine may improve right ventricular function and right ventricular coronary perfusion.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
However, its use should probably be limited to hypotensive patients.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Dobutamine enhances contractility with an increase in stroke volume and cardiac output. However, its systemic vasodilatory effect can lead to hypotension.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Epinephrine combines the beneficial properties of norepinephrine (vasoconstriction with increased right ventricular perfusion, positive inotropy) and dobutamine (positive inotropy), but without the vasodilatory effects associated with the latter.[161]Layish DT, Tapson VF. Pharmacologic hemodynamic support in massive pulmonary embolism. Chest. 1997 Jan;111(1):218-24.
http://www.ncbi.nlm.nih.gov/pubmed/8996020?tool=bestpractice.com
Initiation-phase anticoagulation (5-21 days)
Patients who present with suspected PE should receive an anticoagulant dosed according to the initiation phase of therapy (the initial period following PE diagnosis, lasting 5-21 days depending on the drug selected), unless contraindicated.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[162]Willoughby L, Adams DM, Evans RS, et al. Preemptive anticoagulation in patients with a high pretest probability of pulmonary embolism: are guidelines followed? Chest. 2018 May;153(5):1153-9.
https://www.doi.org/10.1016/j.chest.2017.11.007
http://www.ncbi.nlm.nih.gov/pubmed/29154971?tool=bestpractice.com
If PE is subsequently excluded, anticoagulation can be discontinued. In patients with confirmed PE, anticoagulation should be adjusted to the treatment-phase dose after completion of the initiation phase and should continue for at least 3 months.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
Parenteral anticoagulation
Initiation-phase parenteral anticoagulation should be started in patients with high or intermediate clinical (pre-test) PE probability while awaiting results of diagnostic imaging. If a parenteral anticoagulant (UFH, low molecular weight heparin [LMWH], or fondaparinux) is prescribed, it should overlap with the initiation of a vitamin K antagonist (VKA). Parenteral anticoagulation with UFH for a minimum of 5 days is reserved for patients with high-risk PE in whom primary reperfusion (i.e., systemic thrombolytic therapy or embolectomy) is considered.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
In patients with intermediate-high or intermediate-low risk PE, LMWH or fondaparinux are the preferred parenteral anticoagulants in the initiation phase due to lower rates of major bleeding and heparin-induced thrombocytopenia.
Parenteral anticoagulation can be stopped once a therapeutic international normalized ratio (INR) of 2.0 to 3.0 has been established and at least 5 days overlap with VKA has elapsed.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
UFH has a short half-life, is easy to monitor, and is readily reversed by protamine.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Direct oral anticoagulants (DOACs)
Two of the direct-acting oral anticoagulants, rivaroxaban and apixaban, may be prescribed without the need for pretreatment with a parenteral anticoagulant. However, both dabigatran and edoxaban require lead-in therapy with a parenteral anticoagulant for 5-10 days.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
One meta-analysis found that direct oral anticoagulants have similar efficacy to a heparin/VKA regimen, but with reduced risk of major bleeding (relative risk 0.61, 95% CI 0.21 to 0.68).[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
[163]van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014 Sep 18;124(12):1968-75.
http://www.bloodjournal.org/content/124/12/1968.long
http://www.ncbi.nlm.nih.gov/pubmed/24963045?tool=bestpractice.com
Similarly, a network analysis reported few statistically significant differences between a LMWH/VKA combination and other anticoagulant treatment strategies (for venous thromboembolism [VTE]); however, UFH/VKA may be the least effective strategy, and apixaban initiation may be associated with lowest risk of bleeding.[164]Castellucci LA, Cameron C, Le Gal G, et al. Clinical and safety outcomes associated with treatment of acute venous thromboembolism: a systematic review and meta-analysis. JAMA. 2014 Sep 17;312(11):1122-35.
https://www.doi.org/10.1001/jama.2014.10538
http://www.ncbi.nlm.nih.gov/pubmed/25226478?tool=bestpractice.com
DOACs do not require drug-level or hemostatic monitoring, have a rapid onset of action, and are short-acting. They do not interact with food, but they can interact with other pharmacotherapeutic agents and require serum creatinine monitoring. Dabigatran can be reversed with idarucizumab. Andexanet alfa is approved by the Food and Drug Administration and the European Medicines Agency for the reversal of apixaban- and rivaroxaban-mediated anticoagulation in patients with life-threatening or uncontrolled bleeding. At present there is no approved reversal agent for edoxaban.
There are limited or no data supporting the use of DOACs in conjunction with thrombolytic therapy during the initiation phase. After the patient has been stabilized, clinicians should adopt a shared decision-making process for selecting treatment phase anticoagulation in this patient population, considering both limitations in evidence and pragmatics of care.
Specific patient populations
A VKA is recommended for patients with severe renal impairment (creatinine clearance <30 mL/minute).[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Apixaban is also suitable for use in patients with severe renal dysfunction or end-stage renal disease, though evidence for use in this patient population is limited.[165]Pathak R, Pandit A, Karmacharya P, et al. Meta-analysis on risk of bleeding with apixaban in patients with renal impairment. Am J Cardiol. 2015 Feb 1;115(3):323-7.
https://www.doi.org/10.1016/j.amjcard.2014.10.042
http://www.ncbi.nlm.nih.gov/pubmed/25527282?tool=bestpractice.com
The American College of Chest Physicians (ACCP) recommends apixaban, edoxaban, or rivaroxaban over LMWH for the initiation phase in patients with active cancer (cancer-associated thrombosis).[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
DOACs (particularly edoxaban and rivaxobaran) are associated with a higher risk of gastrointestinal bleeding than LMWH. In patients with luminal gastrointestinal cancer, the ACCP recommends apixaban or LMWH as preferred agents.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[  ]
How do low‐molecular‐weight heparin (LMWH), vitamin K agonists (VKAs), and direct oral anticoagulants (DOACs) compare for treatment of venous thromboembolism (VTE) in people with cancer?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2813/fullShow me the answer The American Society of Hematology (ASH) recommends rivaroxaban, apixaban, or LMWH for initiation-phase anticoagulation in patients with active cancer; ASH acknowledges that this recommendation is conditional based on very low-certainty evidence.[166]Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021 Feb 23;5(4):927-74.
https://ashpublications.org/bloodadvances/article/5/4/927/475194/American-Society-of-Hematology-2021-guidelines-for
http://www.ncbi.nlm.nih.gov/pubmed/33570602?tool=bestpractice.com
]
How do low‐molecular‐weight heparin (LMWH), vitamin K agonists (VKAs), and direct oral anticoagulants (DOACs) compare for treatment of venous thromboembolism (VTE) in people with cancer?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2813/fullShow me the answer The American Society of Hematology (ASH) recommends rivaroxaban, apixaban, or LMWH for initiation-phase anticoagulation in patients with active cancer; ASH acknowledges that this recommendation is conditional based on very low-certainty evidence.[166]Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021 Feb 23;5(4):927-74.
https://ashpublications.org/bloodadvances/article/5/4/927/475194/American-Society-of-Hematology-2021-guidelines-for
http://www.ncbi.nlm.nih.gov/pubmed/33570602?tool=bestpractice.com
LMWH is preferred in patients with severe hepatic impairment and coagulopathy.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Health professionals should refer to the label and/or local formularies before prescribing a DOAC for a patient with renal or hepatic impairment.
Fondaparinux carries a low risk of inducing heparin-induced thrombocytopenia (HIT) and appears to be effective for patients with suspected or confirmed HIT.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[167]Kang M, Alahmadi M, Sawh S, et al. Fondaparinux for the treatment of suspected heparin-induced thrombocytopenia: a propensity score-matched study. Blood. 2015 Feb 5;125(6):924-9.
http://www.bloodjournal.org/content/125/6/924.long
http://www.ncbi.nlm.nih.gov/pubmed/25515959?tool=bestpractice.com
It is contraindicated in patients with severe renal impairment.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Pregnancy
LMWH is recommended for women who are pregnant (weight-adjusted dose) or who may be pregnant.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[168]Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2 Suppl):e691S-736S.
https://journal.chestnet.org/article/S0012-3692(12)60136-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/22315276?tool=bestpractice.com
It does not cross the placenta, and routine monitoring is not generally required.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[169]Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top guideline No. 37a. Apr 2015 [internet publication].
https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf
[170]Bates SM, Rajasekhar A, Middeldorp S, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018 Nov 27;2(22):3317-59.
https://www.doi.org/10.1182/bloodadvances.2018024802
http://www.ncbi.nlm.nih.gov/pubmed/30482767?tool=bestpractice.com
Other anticoagulants, including VKAs, may cross the placenta with attendant risk of fetal adverse effects.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
DOACs are contraindicated in pregnancy.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[171]Cohen H, Arachchillage DR, Middeldorp S, et al. Management of direct oral anticoagulants in women of childbearing potential: guidance from the SSC of the ISTH. J Thromb Haemost. 2016 Aug;14(8):1673-6.
https://www.doi.org/10.1111/jth.13366
http://www.ncbi.nlm.nih.gov/pubmed/27346676?tool=bestpractice.com
Clinical surveillance is recommended over anticoagulation in patients with subsegmental PE (i.e., no involvement of more proximal pulmonary arteries) who do not have proximal deep venous thrombosis (DVT) in the legs, and who are at low risk for recurrent VTE. Clinical surveillance involves informing patients about the clinical signs and symptoms of progressive thrombosis to watch for and the need to be reassessed if these are present.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Primary reperfusion in patients with shock or hypotension
Systemic thrombolytic therapy (preferably with alteplase or reteplase; tenecteplase is an alternative) is recommended in patients with hemodynamic compromise (shock, systolic BP <90 mmHg, or vasopressor requirement to maintain systolic BP >90 mmHg), as this patient group has a high mortality rate.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
[172]Zuo Z, Yue J, Dong BR, et al. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev. 2021 Apr 15;4:CD004437.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004437.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/33857326?tool=bestpractice.com
[173]Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014 Jun 18;311(23):2414-21.
https://jamanetwork.com/journals/jama/fullarticle/1881311
http://www.ncbi.nlm.nih.gov/pubmed/24938564?tool=bestpractice.com
[174]Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020 Oct 13;4(19):4693-738.
https://www.doi.org/10.1182/bloodadvances.2020001830
http://www.ncbi.nlm.nih.gov/pubmed/33007077?tool=bestpractice.com
[175]Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014 Apr 10;370(15):1402-11.
https://www.nejm.org/doi/10.1056/NEJMoa1302097
http://www.ncbi.nlm.nih.gov/pubmed/24716681?tool=bestpractice.com
[176]Kline JA, Hernandez-Nino J, Jones AE. Tenecteplase to treat pulmonary embolism in the emergency department. J Thromb Thrombolysis. 2007 Apr;23(2):101-5.
https://www.doi.org/10.1007/s11239-006-9018-3
http://www.ncbi.nlm.nih.gov/pubmed/17221330?tool=bestpractice.com
The ACCP recommends systemic thrombolytic therapy (unless contraindicated) using a peripheral vein for patients with acute PE associated with hypotension who do not have a high bleeding risk. The ACCP does not make specific recommendations on preferred agents due to lack of comparative data.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Systemic thrombolytic therapy is associated with lower all-cause mortality than anticoagulation alone in patients with high-risk (massive) PE (acute PE with sustained hypotension [i.e., systolic BP <90 mmHg for at least 15 minutes]).[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[172]Zuo Z, Yue J, Dong BR, et al. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev. 2021 Apr 15;4:CD004437.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004437.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/33857326?tool=bestpractice.com
[173]Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014 Jun 18;311(23):2414-21.
https://jamanetwork.com/journals/jama/fullarticle/1881311
http://www.ncbi.nlm.nih.gov/pubmed/24938564?tool=bestpractice.com
[177]Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015 Mar 7;36(10):605-14.
https://academic.oup.com/eurheartj/article/36/10/605/514452
http://www.ncbi.nlm.nih.gov/pubmed/24917641?tool=bestpractice.com
Ideally, PE should be confirmed by imaging before thrombolytic therapy is administered.[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
However, if the patient is at risk of imminent cardiac arrest, treatment may be commenced on clinical grounds alone.[96]British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003 Jun;58(6):470-83.
http://thorax.bmj.com/content/58/6/470.long
http://www.ncbi.nlm.nih.gov/pubmed/12775856?tool=bestpractice.com
Systemic thrombolytic therapy induces prompt clot dissolution and improves right ventricular function, pulmonary blood flow, and lung perfusion.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[177]Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015 Mar 7;36(10):605-14.
https://academic.oup.com/eurheartj/article/36/10/605/514452
http://www.ncbi.nlm.nih.gov/pubmed/24917641?tool=bestpractice.com
Thrombolysis plus heparin was associated with significantly reduced 30-day mortality compared with heparin alone (2.3% [24/1033] vs. 3.9% [40/1024], respectively; pooled odds ratio [OR] 0.59, 95% CI 0.36 to 0.96, P=0.03) in a meta-analysis of patients with acute PE.[177]Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015 Mar 7;36(10):605-14.
https://academic.oup.com/eurheartj/article/36/10/605/514452
http://www.ncbi.nlm.nih.gov/pubmed/24917641?tool=bestpractice.com
Thrombolysis is, however, associated with a significantly increased risk of major and minor bleeding, including hemorrhagic stroke.[172]Zuo Z, Yue J, Dong BR, et al. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev. 2021 Apr 15;4:CD004437.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004437.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/33857326?tool=bestpractice.com
[173]Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014 Jun 18;311(23):2414-21.
https://jamanetwork.com/journals/jama/fullarticle/1881311
http://www.ncbi.nlm.nih.gov/pubmed/24938564?tool=bestpractice.com
[177]Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015 Mar 7;36(10):605-14.
https://academic.oup.com/eurheartj/article/36/10/605/514452
http://www.ncbi.nlm.nih.gov/pubmed/24917641?tool=bestpractice.com
More patients receiving thrombolytic therapy plus heparin experienced a major bleeding episode compared with those taking an anticoagulant alone (9.9% [96/974] vs. 3.6% [35/961], respectively; OR 2.91, 95% CI 1.95 to 4.36).[177]Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015 Mar 7;36(10):605-14.
https://academic.oup.com/eurheartj/article/36/10/605/514452
http://www.ncbi.nlm.nih.gov/pubmed/24917641?tool=bestpractice.com
The reported incidence of intracranial or fatal hemorrhage was 1.7% in the thrombolysis group and 0.3% in the anticoagulant group.[177]Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015 Mar 7;36(10):605-14.
https://academic.oup.com/eurheartj/article/36/10/605/514452
http://www.ncbi.nlm.nih.gov/pubmed/24917641?tool=bestpractice.com
Absolute contraindications to thrombolysis include: hemorrhagic stroke or stroke of unknown origin at any time; ischemic stroke in the preceding 6 months; central nervous system damage or neoplasms; recent major trauma/surgery/head injury (in the preceding 3 weeks); gastrointestinal bleeding within the last month; known bleeding risk.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[178]Van de Werf F, Ardissino D, Betriu A, et al; task force on the management of acute myocardial infarction of the European Society of Cardiology. Management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2003 Jan;24(1):28-66.
https://academic.oup.com/eurheartj/article/24/1/28/562262
http://www.ncbi.nlm.nih.gov/pubmed/12559937?tool=bestpractice.com
Relative contraindications to thrombolysis include: transient ischemic attack in the preceding 6 months; oral anticoagulant therapy; pregnancy, or within 1 week postpartum; traumatic resuscitation (in relation to this episode of PE); refractory hypertension (systolic BP >180 mmHg); advanced liver disease; infective endocarditis; active peptic ulcer.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[178]Van de Werf F, Ardissino D, Betriu A, et al; task force on the management of acute myocardial infarction of the European Society of Cardiology. Management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2003 Jan;24(1):28-66.
https://academic.oup.com/eurheartj/article/24/1/28/562262
http://www.ncbi.nlm.nih.gov/pubmed/12559937?tool=bestpractice.com
Thrombolytic therapy is not typically recommended for hemodynamically stable patients with acute PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
In a randomized double-blind trial, primary reperfusion thrombolytic therapy plus heparin in normotensive patients with intermediate-risk PE (acute right ventricular dysfunction and myocardial injury without overt hemodynamic compromise) prevented hemodynamic decompensation compared with heparin alone, but increased the risk of major hemorrhage and stroke.[175]Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014 Apr 10;370(15):1402-11.
https://www.nejm.org/doi/10.1056/NEJMoa1302097
http://www.ncbi.nlm.nih.gov/pubmed/24716681?tool=bestpractice.com
Surgical embolectomy or catheter-directed therapy
Systemic thrombolytic therapy increases bleeding risk, including that of intracranial bleeding.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[177]Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2015 Mar 7;36(10):605-14.
https://academic.oup.com/eurheartj/article/36/10/605/514452
http://www.ncbi.nlm.nih.gov/pubmed/24917641?tool=bestpractice.com
Surgical pulmonary embolectomy and catheter-directed therapy (which typically involves a combination of mechanical and pharmacotherapeutic thrombus fragmentation) likely have lower attendant bleeding risk than systemic therapy.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[179]Dolovich LR, Ginsberg JS, Douketis JD, et al. A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism: examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Arch Intern Med. 2000 Jan 24;160(2):181-8.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/485206
http://www.ncbi.nlm.nih.gov/pubmed/10647756?tool=bestpractice.com
[180]Konstantinides SV, Barco S. Systemic thrombolytic therapy for acute pulmonary embolism: who is a candidate? Semin Respir Crit Care Med. 2017 Feb;38(1):56-65.
http://www.ncbi.nlm.nih.gov/pubmed/28208199?tool=bestpractice.com
[181]Goldberg JB, Giri J, Kobayashi T, et al. Surgical management and mechanical circulatory support in high-risk pulmonary embolisms: historical context, current status, and future directions: a scientific statement from the American Heart Association. Circulation. 2023 Feb 28;147(9):e628-e47.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001117
http://www.ncbi.nlm.nih.gov/pubmed/36688837?tool=bestpractice.com
Surgical pulmonary embolectomy is recommended for patients in whom systemic thrombolytic therapy has failed or is absolutely contraindicated.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[181]Goldberg JB, Giri J, Kobayashi T, et al. Surgical management and mechanical circulatory support in high-risk pulmonary embolisms: historical context, current status, and future directions: a scientific statement from the American Heart Association. Circulation. 2023 Feb 28;147(9):e628-e47.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001117
http://www.ncbi.nlm.nih.gov/pubmed/36688837?tool=bestpractice.com
Mortality rates following pulmonary embolectomy range from 4% to 27%.[182]Fukuda I, Daitoku K. Surgical embolectomy for acute pulmonary thromboembolism. Ann Vasc Dis. 2017 Jun 25;10(2):107-14.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5579785
http://www.ncbi.nlm.nih.gov/pubmed/29034035?tool=bestpractice.com
In a small cohort of patients who underwent surgical pulmonary embolectomy for acute massive pulmonary thromboembolism, the 10-year survival rate was 84%.[183]Fukuda I, Taniguchi S, Fukui K, et al. Improved outcome of surgical pulmonary embolectomy by aggressive intervention for critically ill patients. Ann Thorac Surg. 2011 Mar;91(3):728-32.
https://www.annalsthoracicsurgery.org/article/S0003-4975(10)02483-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21352987?tool=bestpractice.com
Catheter-directed therapy, which typically involves a combination of mechanical and pharmacotherapeutic thrombus fragmentation, may be considered for patients with acute PE associated with hypotension who also have a high bleeding risk, failed systemic thrombolysis, or shock that is likely to cause death before systemic thrombolysis can take effect (e.g., within hours), if appropriate expertise and resources are available.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Catheter-directed therapy uses a lower dose of thrombolytic drug (approximately one third of full-dose systemic thrombolytic therapy) and is believed to reduce the risks of bleeding at remote sites (e.g., intracranially or gastrointestinally).[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
A meta-analysis of nonrandomized trials of catheter-directed therapies reported a clinical success rate of 87% with an associated risk of major and minor complications of 2% and 8%, respectively.[184]Kuo WT, Gould MK, Louie JD, at al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009 Nov;20(11):1431-40.
http://www.ncbi.nlm.nih.gov/pubmed/19875060?tool=bestpractice.com
Evidence is limited by small studies, study design (i.e., nonrandomized), and use of intermediate efficacy end points.[185]Jimenez D, Martin-Saborido C, Muriel A, et al. Efficacy and safety outcomes of recanalisation procedures in patients with acute symptomatic pulmonary embolism: systematic review and network meta-analysis. Thorax. 2018 May;73(5):464-71.
https://www.doi.org/10.1136/thoraxjnl-2017-210040
http://www.ncbi.nlm.nih.gov/pubmed/29133351?tool=bestpractice.com
Inferior vena cava filters
An inferior vena cava (IVC) filter can be placed in patients:[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[180]Konstantinides SV, Barco S. Systemic thrombolytic therapy for acute pulmonary embolism: who is a candidate? Semin Respir Crit Care Med. 2017 Feb;38(1):56-65.
http://www.ncbi.nlm.nih.gov/pubmed/28208199?tool=bestpractice.com
With acute PE and an absolute contraindication to anticoagulant therapy, such as active major bleeding
With confirmed recurrent PE despite adequate anticoagulation.
ACCP guidelines recommend using an IVC filter only for patients with acute PE (e.g., diagnosed in the preceding 1 month) and an absolute contraindication to anticoagulant therapy (e.g., active major bleeding, severe thrombocytopenia, high bleeding risk, central nervous system lesion). The ACCP recommends against the use of IVC filters in addition to anticoagulation in patients with acute PE.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Other guidelines consider relative indications for IVC filter use to include massive PE with residual deep venous thrombus in a patient at risk for further PE, free-floating iliofemoral or IVC thrombus, and severe cardiopulmonary disease and DVT (e.g., cor pulmonale with pulmonary hypertension).[186]American College of Radiology; Society of Interventional Radiology. ACR-SIR-SPR practice parameter for the performance of inferior vena cava (IVC) filter placement for the prevention of pulmonary embolism. 2021 [internet publication].
https://www.acr.org/-/media/ACR/Files/Practice-Parameters/ivc-fliterplacement.pdf?la=en
Some centers insert IVC filters intraoperatively or immediately postoperatively in patients who undergo surgical pulmonary embolectomy.[187]Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: Results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005 May;129(5):1018-23.
http://www.ncbi.nlm.nih.gov/pubmed/15867775?tool=bestpractice.com
[188]Aklog L, Williams CS, Byrne JG, et al. Acute pulmonary embolectomy: a contemporary approach. Circulation. 2002 Mar 26;105(12):1416-9.
http://circ.ahajournals.org/content/105/12/1416.long
http://www.ncbi.nlm.nih.gov/pubmed/11914247?tool=bestpractice.com
[189]Greelish JP, Leacche M, Solenkova NS, et al. Improved midterm outcomes for type A (central) pulmonary emboli treated surgically. J Thorac Cardiovasc Surg. 2011 Dec;142(6):1423-9.
https://www.jtcvs.org/article/S0022-5223(11)00280-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21481423?tool=bestpractice.com
IVC filter placement should take place as early as possible if it is the only treatment that can be initiated. There is little evidence available to suggest the ideal time for placement. However, the highest risk of dying is within the first 2 hours of presentation, which might indicate that this is a reasonable timeframe for filter placement.[190]Tow DE, Wagner HN. Urokinase pulmonary embolism trial: Phase I results. JAMA. 1970 Dec 21;214(12):2163-72.
https://jamanetwork.com/journals/jama/article-abstract/358702
Observational studies suggest that insertion of a venous filter might reduce PE-related mortality rates in the acute phase but with an associated increase in the risk of filter-related VTE.[191]Stein PD, Matta F, Keyes DC, et al. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med. 2012 May;125(5):478-84.
https://www.amjmed.com/article/S0002-9343(11)00481-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/22310013?tool=bestpractice.com
[192]Muriel A, Jiménez D, Aujesky D, et al. Survival effects of inferior vena cava filter in patients with acute symptomatic venous thromboembolism and a significant bleeding risk. J Am Coll Cardiol. 2014 Apr 29;63(16):1675-83.
http://www.ncbi.nlm.nih.gov/pubmed/24576432?tool=bestpractice.com
Complications associated with permanent IVC filters are common, although they are rarely fatal.[192]Muriel A, Jiménez D, Aujesky D, et al. Survival effects of inferior vena cava filter in patients with acute symptomatic venous thromboembolism and a significant bleeding risk. J Am Coll Cardiol. 2014 Apr 29;63(16):1675-83.
http://www.ncbi.nlm.nih.gov/pubmed/24576432?tool=bestpractice.com
Early complications (including insertion-site thrombosis) occur in approximately 10% of patients. Late complications are more frequent and include recurrent DVT (approximately 20% of patients) and post-thrombotic syndrome (up to 40% of patients).[193]Rajasekhar A, Streiff MB. Vena cava filters for management of venous thromboembolism: a clinical review. Blood Rev. 2013 Sep;27(5):225-41.
http://www.ncbi.nlm.nih.gov/pubmed/23932118?tool=bestpractice.com
[194]PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prévention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005 Jul 19;112(3):416-22.
http://circ.ahajournals.org/content/112/3/416.long
http://www.ncbi.nlm.nih.gov/pubmed/16009794?tool=bestpractice.com
Occlusion of the IVC affects approximately 22% of patients at 5 years and 33% at 9 years, regardless of the use and duration of anticoagulation.[194]PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prévention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005 Jul 19;112(3):416-22.
http://circ.ahajournals.org/content/112/3/416.long
http://www.ncbi.nlm.nih.gov/pubmed/16009794?tool=bestpractice.com
Post-filter anticoagulation should be considered on a case-by-case basis according to relative and absolute contraindications.[195]Decousus H, Leizorovicz A, Parent F, et al; Prévention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med. 1998 Feb 12;338(7):409-15.
https://www.nejm.org/doi/10.1056/NEJM199802123380701
http://www.ncbi.nlm.nih.gov/pubmed/9459643?tool=bestpractice.com
Anticoagulation should be initiated if the contraindication resolves or if a risk/benefit analysis suggests this to be a reasonable course.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
When retrievable filters are used, they should be removed as soon as it is safe to use anticoagulants.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Prognostic stratification of patients with confirmed PE without shock or hypotension: Pulmonary Embolism Severity Index
Patients with confirmed PE without shock or hypotension require further risk stratification, for example with the Pulmonary Embolism Severity Index (PESI) or the simplified Pulmonary Embolism Severity Index (sPESI).[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
PESI classifies patients with confirmed PE without shock or hypotension into 1 of 5 risk categories associated with increasing 30-day mortality. PESI risk category is derived from the sum of points for 11 clinical criteria; sPESI has only 6 criteria, and reports risk stratification dichotomously (low [0 points] or high [≥1 point(s)] risk of 30-day mortality).
Studies indicate that PESI and sPESI predict short-term mortality with comparable accuracy, but the latter is easier to use.[196]Vinson DR, Ballard DW, Mark DG, et al; MAPLE Investigators of the KP CREST Network. Risk stratifying emergency department patients with acute pulmonary embolism: does the simplified Pulmonary Embolism Severity Index perform as well as the original? Thromb Res. 2016 Dec;148:1-8.
http://www.ncbi.nlm.nih.gov/pubmed/27764729?tool=bestpractice.com
[197]Zhou XY, Ben SQ, Chen HL, et al. The prognostic value of pulmonary embolism severity index in acute pulmonary embolism: a meta-analysis. Respir Res. 2012 Dec 4;13:111.
https://respiratory-research.biomedcentral.com/articles/10.1186/1465-9921-13-111
http://www.ncbi.nlm.nih.gov/pubmed/23210843?tool=bestpractice.com
One meta-analysis that evaluated the prognostic utility of PESI/sPESI for all-cause mortality reported pooled sensitivity and pooled specificity of 91% and 41%, respectively.[197]Zhou XY, Ben SQ, Chen HL, et al. The prognostic value of pulmonary embolism severity index in acute pulmonary embolism: a meta-analysis. Respir Res. 2012 Dec 4;13:111.
https://respiratory-research.biomedcentral.com/articles/10.1186/1465-9921-13-111
http://www.ncbi.nlm.nih.gov/pubmed/23210843?tool=bestpractice.com
PESI has been used to identify patients eligible for outpatient management in prospective studies.[198]Roy PM, Moumneh T, Penaloza A, et al. Outpatient management of pulmonary embolism. Thromb Res. 2017 Jul;155:92-100.
http://www.ncbi.nlm.nih.gov/pubmed/28525830?tool=bestpractice.com
[199]Bledsoe JR, Woller SC, Stevens SM, et al. Management of Low-Risk Pulmonary Embolism Patients Without Hospitalization: The Low-Risk Pulmonary Embolism Prospective Management Study. Chest. 2018 Aug;154(2):249-256.
https://www.doi.org/10.1016/j.chest.2018.01.035
http://www.ncbi.nlm.nih.gov/pubmed/29410163?tool=bestpractice.com
Based upon social background and likely compliance with treatment, European Society of Cardiology guidelines suggest that low-risk patients (PESI class I or class II), and potentially those with a sPESI score of 0, can be considered for early discharge and outpatient management.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[199]Bledsoe JR, Woller SC, Stevens SM, et al. Management of Low-Risk Pulmonary Embolism Patients Without Hospitalization: The Low-Risk Pulmonary Embolism Prospective Management Study. Chest. 2018 Aug;154(2):249-256.
https://www.doi.org/10.1016/j.chest.2018.01.035
http://www.ncbi.nlm.nih.gov/pubmed/29410163?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: PESI criteria and risk stratificationCreated by BMJ Knowledge Centre [Citation ends]. [Figure caption and citation for the preceding image starts]: sPESI criteria and risk stratificationCreated by BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: sPESI criteria and risk stratificationCreated by BMJ Knowledge Centre [Citation ends].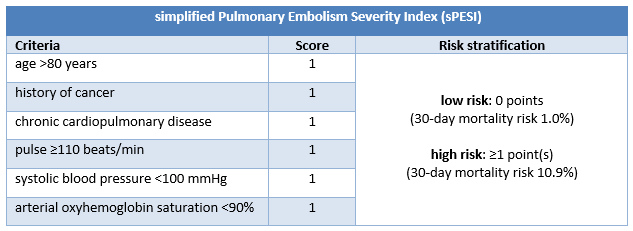
Rescue thrombolysis
Right ventricular function assessed by echocardiography and cardiac troponin testing should be considered in patients with PESI risk stratification ≥III or sPESI ≥1.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
In patients with confirmed PE without shock or hypotension: right ventricular dysfunction is predictive of adverse outcome and enables further risk stratification; elevated troponin levels are associated with increased risk for short-term mortality, PE-related mortality, and serious adverse events.[130]Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med. 2009 Mar;122(3):257-64.
http://www.ncbi.nlm.nih.gov/pubmed/19272487?tool=bestpractice.com
[131]Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011 Apr 26;123(16):1788-830.
http://circ.ahajournals.org/content/123/16/1788.long
http://www.ncbi.nlm.nih.gov/pubmed/21422387?tool=bestpractice.com
[132]Weekes AJ, Thacker G, Troha D, et al. Diagnostic accuracy of right ventricular dysfunction markers in normotensive emergency department patients with acute pulmonary embolism. Ann Emerg Med. 2016 Sep;68(3):277-91.
http://www.ncbi.nlm.nih.gov/pubmed/26973178?tool=bestpractice.com
[200]Bajaj A, Saleeb M, Rathor P, et al. Prognostic value of troponins in acute nonmassive pulmonary embolism: a meta-analysis. Heart Lung. 2015 Jul-Aug;44(4):327-34.
http://www.ncbi.nlm.nih.gov/pubmed/25976228?tool=bestpractice.com
[201]Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007 Jul 24;116(4):427-33.
http://circ.ahajournals.org/content/116/4/427.long
http://www.ncbi.nlm.nih.gov/pubmed/17606843?tool=bestpractice.com
Intermediate high-risk patients
Patients with PESI risk stratification ≥III, or sPESI ≥1, with right ventricular dysfunction and a positive cardiac troponin test belong to an intermediate high-risk group.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Rescue thrombolysis may be indicated in intermediate high-risk patients, and in patients with other clinical features of cardiopulmonary impairment (e.g., elevated heart rate, respiratory rate, jugular venous pressure) who have started anticoagulant therapy, and:[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[180]Konstantinides SV, Barco S. Systemic thrombolytic therapy for acute pulmonary embolism: who is a candidate? Semin Respir Crit Care Med. 2017 Feb;38(1):56-65.
http://www.ncbi.nlm.nih.gov/pubmed/28208199?tool=bestpractice.com
Are deteriorating (as seen by a decrease in systolic BP, increase in heart rate, worsening gas exchange, signs of inadequate perfusion, worsening RV function, or increasing cardiac biomarkers), but have not yet developed hypotension[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Are exhibiting signs of hemodynamic decompensation (e.g., systolic BP <90 mmHg for at least 15 minutes, or drop of systolic BP by at least 40 mmHg for at least 15 minutes with signs of end organ hypoperfusion).
Consideration of bleeding risk will inform choice of thrombolytic therapy.
Intermediate low-risk patients
Normotensive patients with PESI risk stratification ≥III, or sPESI ≥1, with normal echocardiogram and/or cardiac troponin test are considered to be intermediate low-risk.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Intermediate low-risk patients should be monitored if the cardiac troponin test is positive (even in the absence of right ventricular dysfunction); anticoagulant therapy should be maintained in all intermediate-low risk patients.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Other prognostic indices
The RIETE (Registro Informatizado de la Enfermedad TromboEmbolica venosa) and HESTIA criteria may be helpful in selecting patients (with VTE at low risk of adverse clinical outcome) who could be managed as outpatients.[202]Trujillo-Santos J, Lozano F, Lorente MA, et al. A prognostic score to identify low-risk outpatients with acute deep vein thrombosis in the lower limbs. Am J Med. 2015 Jan;128(1):90.e9-15.
http://www.ncbi.nlm.nih.gov/pubmed/25242230?tool=bestpractice.com
[203]Zondag W, Vingerhoets LM, Durian MF, et al. Hestia criteria can safely select patients with pulmonary embolism for outpatient treatment irrespective of right ventricular function. J Thromb Haemost. 2013 Apr;11(4):686-92.
https://onlinelibrary.wiley.com/doi/full/10.1111/jth.12146
http://www.ncbi.nlm.nih.gov/pubmed/23336721?tool=bestpractice.com
[204]Weeda ER, Kohn CG, Peacock WF, et al. External validation of the Hestia criteria for identifying acute pulmonary embolism patients at low risk of early mortality. Clin Appl Thromb Hemost. 2017 Oct;23(7):769-74.
http://journals.sagepub.com/doi/pdf/10.1177/1076029616651147
http://www.ncbi.nlm.nih.gov/pubmed/27225840?tool=bestpractice.com
Treatment-phase anticoagulant therapy
ACCP guidelines recommend that patients who do not have a contraindication are given a 3-month treatment phase of anticoagulation. Apixaban, dabigatran, edoxaban, or rivaroxaban are recommended over a VKA.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Once the treatment phase is complete, all patients should be evaluated for extended-phase therapy.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Extended-phase anticoagulant therapy
The goal for continuation of anticoagulant therapy into the extended phase (i.e., beyond the first 3 months and with no scheduled stop date) is secondary prevention of VTE.
ACCP guidelines recommend that the following patients are given extended-phase anticoagulation:[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Those with PE diagnosed in the absence of transient provocation (unprovoked PE or PE provoked by a persistent risk factor). These patients should be given a DOAC
Those with PE diagnosed in the absence of transient risk factor (unprovoked PE or PE provoked by a persistent risk factor) who cannot receive a DOAC. These patients should be given a VKA.
Extended-phase anticoagulation is not recommended in patients with PE diagnosed in the context of a major or a minor transient risk factor.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
The decision to start or continue extended therapy should be based on patient preference and the predicted risk of recurrent VTE or bleeding.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Precise prediction of the risk for recurrent VTE after an initial event is challenging. The presence or absence of temporary provoking factors is the most potent predictor for recurrence. Patients with a major transient provocation (e.g., surgery) within the 3 months prior to the VTE event have the lowest risk for recurrence. Minor transient risk factors (e.g., medical hospitalization, estrogen use, long-haul travel) within 2 months of the diagnosis predict an intermediate risk for recurrence. Patients with no identifiable risk factor for VTE (i.e., unprovoked VTE) or a persisting provocation (e.g., cancer) are at high risk for recurrence.[20]Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016 Jul;14(7):1480-3.
https://www.doi.org/10.1111/jth.13336
http://www.ncbi.nlm.nih.gov/pubmed/27428935?tool=bestpractice.com
Patients with recurrent unprovoked PE and proximal DVT are thought to be at an especially high risk for further recurrence.[174]Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020 Oct 13;4(19):4693-738.
https://www.doi.org/10.1182/bloodadvances.2020001830
http://www.ncbi.nlm.nih.gov/pubmed/33007077?tool=bestpractice.com
When assessing bleeding risk, the following factors should be considered: age >65 years (particularly >75 years), previous bleeding, cancer, renal failure, liver failure, thrombocytopenia, previous stroke, diabetes mellitus, anemia, antiplatelet therapy, poor anticoagulant control, comorbidity with reduced functional capacity, recent surgery, frequent falls, alcohol abuse, use of nonsteroidal anti-inflammatory drugs.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Patients with none of these risk factors are considered low risk; one risk factor renders a patient moderate risk; and two or more risk factors renders a patient high risk.
Several risk prediction models have attempted to identify patients with unprovoked and/or minor transient provocation who have a low risk of recurrent VTE. Of these, the HER-DOO2 model has been evaluated in a number of prospective clinical validation studies and has been used to identify a subpopulation of patients with a low risk of recurrence after stopping anticoagulation therapy (following completion of the treatment phase).[205]Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017 Mar 17;356:j1065.
https://www.doi.org/10.1136/bmj.j1065
http://www.ncbi.nlm.nih.gov/pubmed/28314711?tool=bestpractice.com
Choice of agent
In patients who receive extended-phase anticoagulation therapy, there is usually no need to change the initial oral anticoagulant. ACCP guidelines recommend using reduced-dose apixaban or rivaroxaban for patients receiving apixaban or rivaroxaban; the choice of a particular drug and dose should take into account the patient’s body mass index, renal function, and expected adherence to the dosing regimen.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Lower doses of both apixaban and rivaroxaban are similarly effective for extended-phase anticoagulant therapy, and are associated with a modest reduction in non-major bleeding compared with treatment doses.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[206]Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013 Feb 21;368(8):699-708.
https://www.doi.org/10.1056/NEJMoa1207541
http://www.ncbi.nlm.nih.gov/pubmed/23216615?tool=bestpractice.com
[207]Weitz JI, Lensing AW, Prins MH, et al; EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017 Mar 30;376(13):1211-22.
https://www.nejm.org/doi/10.1056/NEJMoa1700518
http://www.ncbi.nlm.nih.gov/pubmed/28316279?tool=bestpractice.com
Evidence from studies of ≥6 months' duration suggests that there are no differences between direct oral anticoagulants and conventional anticoagulation for the management of PE.[208]Li M, Li J, Wang X, et al. Oral direct thrombin inhibitors or oral factor Xa inhibitors versus conventional anticoagulants for the treatment of pulmonary embolism. Cochrane Database Syst Rev. 2023 Apr 14;4(4):CD010957.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010957.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/37057837?tool=bestpractice.com
[209]Becattini C, Agnelli G. Risk stratification and management of acute pulmonary embolism. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):404-12.
http://asheducationbook.hematologylibrary.org/content/2016/1/404.long
http://www.ncbi.nlm.nih.gov/pubmed/27913508?tool=bestpractice.com
[  ]
How do oral direct thrombin inhibitors (DTIs) and oral factor Xa inhibitors compare with conventional anticoagulation for the treatment of pulmonary embolism?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4357/fullShow me the answer[Evidence B]8457c6d8-a7cb-4512-87ef-044324167df3ccaBHow do oral direct thrombin inhibitors and oral factor Xa inhibitors compare with conventional anticoagulation for the treatment of pulmonary embolism?
]
How do oral direct thrombin inhibitors (DTIs) and oral factor Xa inhibitors compare with conventional anticoagulation for the treatment of pulmonary embolism?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4357/fullShow me the answer[Evidence B]8457c6d8-a7cb-4512-87ef-044324167df3ccaBHow do oral direct thrombin inhibitors and oral factor Xa inhibitors compare with conventional anticoagulation for the treatment of pulmonary embolism?
The continued use of extended-phase anticoagulation should be reassessed at least annually, and at any time there is a significant change in the patient’s clinical status.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
The evidence to continue extended therapy beyond 4 years is uncertain. ACCP guidelines recommend shared decision-making, taking into account the patient values and preferences.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Patients should be periodically reassessed for bleeding risk, burdens of therapy, and any change in values and preferences.
If the decision is to stop extended-phase anticoagulation, ACCP guidelines recommend giving aspirin (unless contraindicated) to prevent recurrent VTE.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
The benefits of using aspirin should be balanced against risk of bleeding and inconvenience of use. Aspirin should not be considered a reasonable alternative for patients who are willing to undergo extended anticoagulation therapy, as aspirin is much less effective. The use of aspirin should always be reassessed when a patient stops anticoagulant therapy (because aspirin might have been stopped when anticoagulant therapy was started).[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Active cancer
For patients with active cancer, ACCP guidelines recommend apixaban, edoxaban, or rivaroxaban over LMWH for extended-phase anticoagulation.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
DOACs (particularly edoxaban and rivaxobaran) are associated with a higher risk of gastrointestinal bleeding than LMWH. In patients with luminal gastrointestinal cancer, the ACCP recommends apixaban or LMWH as preferred agents.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[  ]
How do low‐molecular‐weight heparin (LMWH), vitamin K agonists (VKAs), and direct oral anticoagulants (DOACs) compare for treatment of venous thromboembolism (VTE) in people with cancer?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2813/fullShow me the answer ASH recommends using DOACs or LMWH for the extended phase; ASH acknowledges that this recommendation is conditional based on very low-certainty evidence.[166]Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021 Feb 23;5(4):927-74.
https://ashpublications.org/bloodadvances/article/5/4/927/475194/American-Society-of-Hematology-2021-guidelines-for
http://www.ncbi.nlm.nih.gov/pubmed/33570602?tool=bestpractice.com
]
How do low‐molecular‐weight heparin (LMWH), vitamin K agonists (VKAs), and direct oral anticoagulants (DOACs) compare for treatment of venous thromboembolism (VTE) in people with cancer?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2813/fullShow me the answer ASH recommends using DOACs or LMWH for the extended phase; ASH acknowledges that this recommendation is conditional based on very low-certainty evidence.[166]Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021 Feb 23;5(4):927-74.
https://ashpublications.org/bloodadvances/article/5/4/927/475194/American-Society-of-Hematology-2021-guidelines-for
http://www.ncbi.nlm.nih.gov/pubmed/33570602?tool=bestpractice.com
Extended-phase anticoagulant therapy is recommended in these patients (i.e., no scheduled stop date) while cancer remains active.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[166]Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021 Feb 23;5(4):927-74.
https://ashpublications.org/bloodadvances/article/5/4/927/475194/American-Society-of-Hematology-2021-guidelines-for
http://www.ncbi.nlm.nih.gov/pubmed/33570602?tool=bestpractice.com
Pregnancy
Anticoagulant therapy should be administered for at least 6 weeks postpartum in women at high risk for postpartum VTE, and for a minimum overall treatment duration of 3 months from initial PE diagnosis.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[169]Royal College of Obstetricians and Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top guideline No. 37a. Apr 2015 [internet publication].
https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf
LMWH is used during the antepartum as other anticoagulants, including VKAs, may cross the placenta with attendant risk of fetal adverse effects.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Continued LMWH or VKAs are an option for breast-feeding mothers. DOACs are not recommended for breast-feeding mothers because they are excreted in breast milk, and safety to the nursing baby has not been established.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Severe renal impairment
The ACCP recommends a VKA for patients with severe renal impairment (i.e., creatinine clearance <30 mL/minute).[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Apixaban is also suitable for use in patients with severe renal dysfunction or end-stage renal disease, though evidence for use in this patient population is limited.[165]Pathak R, Pandit A, Karmacharya P, et al. Meta-analysis on risk of bleeding with apixaban in patients with renal impairment. Am J Cardiol. 2015 Feb 1;115(3):323-7.
https://www.doi.org/10.1016/j.amjcard.2014.10.042
http://www.ncbi.nlm.nih.gov/pubmed/25527282?tool=bestpractice.com
Hepatic impairment and coagulopathy
ACCP guidelines recommend LMWH in this patient population.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Health professionals should refer to the label and/or local formularies before prescribing a direct oral anticoagulant for a patient with hepatic impairment.
Antiphospholipid syndrome
In patients with antiphospholipid syndrome, the ACCP and the International Society on Thrombosis and Haemostasis guidelines recommend a VKA as the preferred agent.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[210]Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020 Sep;18(9):2126-37.
https://www.doi.org/10.1111/jth.14935
http://www.ncbi.nlm.nih.gov/pubmed/32881337?tool=bestpractice.com
Evidence from randomized controlled trials suggests that DOACs may not be as effective as VKAs for the treatment of thrombosis among patients with antiphospholipid syndrome. Therefore, ACCP guidelines recommend avoiding DOACs in these patients.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Patients with recurrent VTE on anticoagulant therapy
Recurrent VTE is unusual among patients receiving therapeutic-dose anticoagulant therapy, with the exception of cancer (7% to 9% on-therapy recurrence with LMWH).[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[211]Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015 Sep;136(3):582-9.
https://www.doi.org/10.1016/j.thromres.2015.07.011
http://www.ncbi.nlm.nih.gov/pubmed/26210891?tool=bestpractice.com
In addition to definitively establishing the presence of recurrent PE, consideration should be given to compliance with anticoagulant therapy or the presence of underlying malignancy.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
ACCP guidelines recommend a temporary switch to LMWH (for at least 1 month) for patients with recurrent PE who are thought to be compliant with a non-LMWH anticoagulant (or within the therapeutic range if receiving VKA therapy).[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
An increased dose of LMWH (one quarter to one third) is appropriate for patients with recurrent PE who have been receiving LMWH.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Recurrent VTE following discontinuation of anticoagulant therapy
For patients who are no longer receiving anticoagulant therapy and experience a second PE with no identifiable risk factor (i.e., unprovoked), guidelines recommend the following anticoagulant treatment durations:[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
 ]
The American Society of Hematology (ASH) recommends rivaroxaban, apixaban, or LMWH for initiation-phase anticoagulation in patients with active cancer; ASH acknowledges that this recommendation is conditional based on very low-certainty evidence.[166]
]
The American Society of Hematology (ASH) recommends rivaroxaban, apixaban, or LMWH for initiation-phase anticoagulation in patients with active cancer; ASH acknowledges that this recommendation is conditional based on very low-certainty evidence.[166] [Figure caption and citation for the preceding image starts]: sPESI criteria and risk stratificationCreated by BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: sPESI criteria and risk stratificationCreated by BMJ Knowledge Centre [Citation ends].
 ]
[Evidence B]
]
[Evidence B] ]
ASH recommends using DOACs or LMWH for the extended phase; ASH acknowledges that this recommendation is conditional based on very low-certainty evidence.[166]
]
ASH recommends using DOACs or LMWH for the extended phase; ASH acknowledges that this recommendation is conditional based on very low-certainty evidence.[166]