Tests
1st tests to order
Wells criteria/Geneva score
Test
Should be calculated in patients with suspected PE.
Each of these clinical decision tools assigns a value (a single point, or points) to a series of historic and physical examination features, the sum of which determines whether PE is likely or unlikely.
Sensitivity ranged from 88% (simplified revised Geneva) to 96% (simplified Wells) and specificity from 48% (revised Geneva) to 53% (simplified revised Geneva) when each clinical decision tool (original Wells, modified Wells, simplified Wells, revised Geneva, and simplified revised Geneva; all with D-dimer testing if PE unlikely) was validated in a primary care dataset.[101]
The simplified versions of the modified Wells criteria or revised Geneva score may be preferred in clinical practice because of their ease of use.[100] Both simplified versions have been validated; neither has been shown to be superior to the other.[87][101] However, the Geneva score is based entirely on objective clinical items and may be more reproducible (the Wells criteria [original and simplified] include the subjective clinical item "alternative diagnosis less likely than PE").[102]
Clinical (pretest) probability score alone is not enough to diagnose or exclude PE. It must be combined with additional tests.
[ Pulmonary Embolism Wells Score Opens in new window ]
[
Revised Geneva Score for Estimation of the Clinical Probability of Pulmonary Embolism in Adults
Opens in new window
]
[Figure caption and citation for the preceding image starts]: Original and simplified Wells criteria (modified)Created by the BMJ Knowledge Centre [Citation ends]. [Figure caption and citation for the preceding image starts]: Original and simplified Geneva score (revised)Created by the BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Original and simplified Geneva score (revised)Created by the BMJ Knowledge Centre [Citation ends].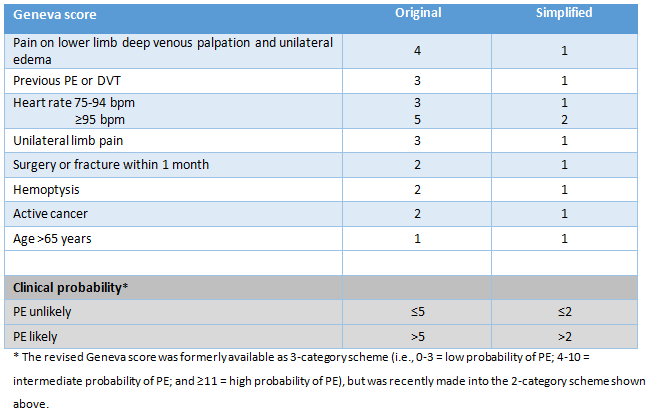
Result
original Wells criteria: PE unlikely ≤4, PE likely >4; simplified Wells criteria: PE unlikely ≤1, PE likely >1; revised Geneva score: PE unlikely ≤5, PE likely >5; simplified Geneva score: PE unlikely ≤2, PE likely >2
multiple-detector computed tomographic pulmonary angiography (CTPA)
Test
Ordered for patients with a PE likely clinical (pretest) probability or D-dimer above threshold.[4][87]
CTPA has the best diagnostic accuracy of all advanced noninvasive imaging methods.[111] The likelihood ratio to rule in a PE with a filling defect in the segmental or subsegmental branches is 24.1 (range of 12.4 to 46.7), whereas the likelihood to rule it out is 0.11 (range of 0.06 to 0.19).[111]
Specificity is 96%.[137] Three-month incidence of a subsequent venous thromboembolic event following a negative CT scan is <2%.[111][138]
CT scans are contraindicated in approximately 25% of patients due to contrast allergy or renal failure.[137] However, radiation exposure through CT scan is at a dose much lower than the exposure associated with fetal harm and, if necessary, should not be withheld from pregnant patients.[139]
CTPA should be used with discretion in children and adolescents, and alternative imaging strategies employed where possible.[135][140] If it is used, ionizing radiation exposure should be carefully monitored and minimized.[141]
[Figure caption and citation for the preceding image starts]: Contrasted CTPA scan showing subsegmental right pulmonary artery emboli (see arrows)From the collection of Seth W. Clemens; used with permission [Citation ends].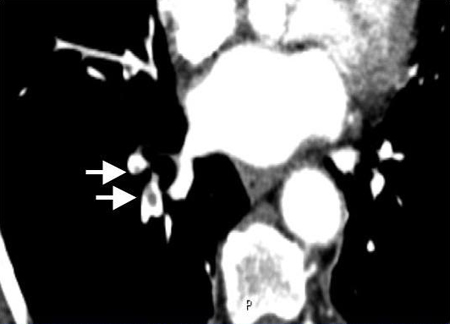
Result
diagnosis is confirmed by direct visualization of thrombus in a pulmonary artery; appears as a partial or complete intraluminal filling defect
ventilation-perfusion (V/Q) scan
Test
V/Q lung scan, preferably using single photon emission computed tomography (SPECT, which may reduce the number of inconclusive scans), is an alternative to CTPA.[112]
A prospective study of patients with suspected acute PE reported a sensitivity of 97% and a specificity of 88% for V/Q SPECT scan.[142] In a retrospective study of 2328 patients with clinically suspected PE, 601 of 608 patients with a final diagnosis of PE had a positive V/Q SPECT scan (99% sensitivity), and 1153 patients without final PE diagnosis had a negative V/Q SPECT scan (98% specificity).[143]
V/Q scan is a radiation- and medium-sparing procedure and may be appropriate for patients with contraindications or relative contraindications to CT (e.g., contrast allergy, moderate to severe renal failure, pregnancy, young patients).[4]
Result
PE likely when an area of ventilation is not perfused; a negative V/Q scan effectively excludes PE
coagulation studies
Test
International normalized ratio (INR), prothrombin time (PT), and activated partial thromboplastin time (aPTT) should be ordered.
Required to establish baseline prior to commencing anticoagulation.
Aids decisions about the safety and type of initial anticoagulation to prescribe.
Result
baseline values; establishing correct therapeutic range
BUN and creatinine, hepatic function tests
Test
The choice of anticoagulant and doses of some anticoagulants may need to be adjusted in patients with renal or hepatic impairment. Baseline values should be obtained.
Result
baseline values; decision on most appropriate anticoagulant and dose
CBC
Test
May indicate thrombocytopenia.
Heparin therapy can be associated with heparin-induced thrombocytopenia; platelet count should be measured at baseline and regularly throughout heparin treatment.
Elevated platelet count may suggest essential thrombocytosis or a myeloproliferative disorder, which may represent a secondary hypercoagulable state.
May detect anemia, leukopenia.
Result
baseline values
Pulmonary Embolism Rule-Out Criteria (PERC)
Test
Guidelines from the American College of Physicians recommend the application of the Pulmonary Embolism Rule-Out Criteria (PERC) to exclude PE in patients initially assessed to have a very low pretest probability of PE.[87]
In patients who meet all PERC criteria (age <50 years; initial heart rate <100 bpm; initial oxygen saturation >94% on room air; no unilateral leg swelling; no hemoptysis; no surgery or trauma within the last 4 weeks; no history of venous thromboembolism; no estrogen use), the risk for PE is considered to be lower than the risk of testing, and no further testing is indicated. Patients who do not meet all of the PERC criteria can be stratified using D-dimer testing.[87]
One meta-analysis of studies that assessed the accuracy of PERC to rule out PE reported a sensitivity of 97%.[104]
Result
fail to meet all criteria (positive score)
D-dimer test
Test
D-dimer testing is indicated in: hemodynamically stable patients with nonhigh clinical probability of PE; patients who are assessed using the Pulmonary Embolism Rule-Out Criteria (PERC) score and who do not meet all its criteria (patients meeting all PERC criteria are at sufficiently low risk for PE that D-dimer test need not be performed); patients whose initial risk stratification identifies them as being at low risk of PE and who did not undergo assessment with PERC.[4][87]
Clinicians should not obtain a D-dimer measurement in patients with a high clinical probability of PE; immediate imaging is indicated.[87]
D-dimer testing is highly sensitive (>95%) but nonspecific.
A plasma D-dimer level below threshold safely excludes PE in patients with an unlikely (intermediate or low) pretest probability of PE, and no further testing is required.[87][95] The risk of PE within 3 months is <1% in these patients.[105][106]
D-dimer may be adjusted to age (normal is <age x 10 micrograms/L in patients 50 years or older) or pre-test probability of disease (1000 nanograms/mL cutoff in low-probability patients, or 500 nanograms/mL in intermediate probability patients) to increase the specificity, and thus the percentage of patients who can avoid an imaging study.[4][107][108]
In patients with cancer, using an age-adjusted D-dimer cut-off doubled the proportion of patients in whom PE could be excluded by clinical decision rule and D-dimer, without imaging.[109] The YEARS algorithm with risk-adapted D-dimer thresholds has been studied in pregnant patients suspected of PE.[110]
Result
elevated
Tests to consider
chest x-ray
Test
A normal chest x-ray does not eliminate PE as a diagnosis[115] However, a chest-ray may rule out other causes of a patient’s symptoms such as pneumothorax or pneumonia.[115]
May show Fleischner sign/prominent central pulmonary artery (20%); Westermark sign/oligemia in PE area of distribution (11%); Hampton hump/pleural-based areas of increased opacity corresponding to the distribution of the PE (27%).[99][127][129][144]
Chest x-ray is the first radiation-associated procedure if PE is suspected in pregnancy.[134]
Result
band atelectasis, elevation of hemidiaphragm, prominent central pulmonary artery, oligemia at site of embolism
magnetic resonance (MR) angiography
Test
MR based imaging is rarely used due to the ease and performance characteristics of CTPA.
Three different techniques are available: gadolinium-contrast enhanced angiography (Gd-MRA), real-time angiography (RT-MRA), and MR-perfusion images.[116][Figure caption and citation for the preceding image starts]: Gd-MRA showing a right main pulmonary artery pulmonary embolism (see arrow)From the collection of Seth W. Clemens; used with permission [Citation ends].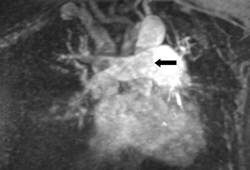
Can be used to evaluate the central and segmental arteries.[115]
High specificity (91% to 98%) allows for accurate diagnosis.
Low sensitivity (75% to 93%) cannot reliably exclude PE with a negative test.[116]
Gadolinium contrast, used in Gd-MRA and MR perfusion studies, is a relative contraindication in pregnancy.
Result
diagnosis is confirmed by direct visualization of thrombus in a pulmonary artery; appears as a partial or complete intraluminal filling defect
pulmonary angiography
Test
Despite its diagnostic accuracy, pulmonary angiography is rarely used for the diagnosis or exclusion of PE.[4][118] It is associated with risk of morbidity/mortality, and (less invasive) CTPA affords comparable diagnostic precision.[4][119]
Negative predictive value may be as high as 99%.[145]
Invasive test with morbidity of 3% to 6% and a mortality of 0.2% to 0.5%.[119][146]
Involves the use of contrast; a relative contraindication in pregnancy and renal failure.
Result
diagnosis made by visualization of a complete or incomplete filling defect in the pulmonary artery
electrocardiography (ECG)
Test
ECG cannot definitively establish or eliminate PE as a diagnosis, and specific findings may only be suggestive of PE.[89][128][129]
ECG can, however, be used to assess right ventricular function in patients with confirmed PE without shock or hypotension.[4][21] Right ventricular dysfunction is predictive of adverse outcome and enables risk stratification in these patients.[130][131][132]
If a definitive imaging modality is unavailable, echocardiography may be considered for patients with suspected PE presenting with shock or hypotension.[4][95]
Result
right ventricular dysfunction; right ventricular enlargement
arterial blood gases (ABG)
Test
ABG analysis is of very limited diagnostic utility, alone or in combination with other clinical variables, in suspected PE.[126]
In patients with suspected acute PE with normal ABG results, PE could not be excluded in 38% of those without cardiopulmonary disease and 14% with pre-existing cardiopulmonary disease, respectively.[127]
Result
hypoxia and hypocapnia may be suggestive
thrombophilia screen
Test
Hereditary thrombophilia does not sufficiently modify the predicted risk of recurrent thrombosis to affect treatment decisions, and a conservative approach to testing is reasonable.[21] Some guidelines suggest testing only in situations where the result is likely to change a clinical decision (such as in patients with unprovoked deep vein thrombosis or pulmonary embolism who are considering stopping anticoagulants).[22]
Controversy exists regarding whether broad screening for antiphospholipid antibodies or screening only on the basis of clinical suspicion should be preferred.[124][125]
Result
positive in inherited thrombophilias; elevated titers/abnormal result in antiphospholipid syndrome
ultrasonography
YEARS criteria
Test
Diagnostic algorithm to rule out PE; incorporates differential D-dimer cutoff values at presentation. May result in reduced need for CTPA, but few validating studies have been published.
The YEARS criteria are: clinical signs of deep vein thrombosis; hemoptysis; and whether PE is the most likely diagnosis.[147]
The YEARS criteria have been evaluated in pregnant patients suspected of PE.
Clinical (pretest) probability score alone is not enough to diagnose or exclude PE. It must be combined with additional tests.
Result
PE excluded when none of the YEARS criteria are met and the D-dimer level is <1000 ng/mL, or ≥1 of the YEARS criteria are met and the D-dimer level is <500 ng/mL
troponin
Test
Troponin can be used to assist in determining the severity category of acute PE, which impacts management decisions.
It is suggested in patients who have either an elevated Pulmonary Embolism Severity Index (PESI) category, or abnormalities of the right ventricle on imaging.[4]
Result
may be elevated; may be useful for prognosis
Use of this content is subject to our disclaimer