Management of PE is based on an algorithmic diagnostic approach. History and physical examination are relatively insensitive and nonspecific, so must be combined with other diagnostic tests in the clinical decision-making process.
A high index of suspicion and prompt management are required as the highest risk of dying is within the first 2 hours of presentation.[8]Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995 Oct;108(4):978-81.
http://www.ncbi.nlm.nih.gov/pubmed/7555172?tool=bestpractice.com
[86]Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013 Spring;18(2):129-38.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3718593
http://www.ncbi.nlm.nih.gov/pubmed/23940438?tool=bestpractice.com
However, PE is present in only a small minority of patients in whom it is suspected.
Clinical probability
Clinical probability, assessed by a validated prediction rule and/or clinical judgment, is the basis for all diagnostic strategies for PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
In hemodynamically stable patients with non-high (low or intermediate) clinical probability of PE, D-dimer measurement is recommended to assess the need for imaging.[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
In patients with very low clinical probability of PE, D-dimer testing is reserved for those who do not meet all of the Pulmonary Embolism Rule-Out Criteria (PERC).[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
Those with a high clinical probability of PE, or with an abnormal D-dimer, should proceed immediately to computed tomographic pulmonary angiography (CTPA; or ventilation-perfusion [V/Q] lung scan if CTPA is contraindicated), as should any patient with suspected PE with shock or hypotension. In patients with high pretest probability of PE, anticoagulation should be initiated while awaiting imaging results.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
[22]National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Aug 2023 [internet publication].
https://www.nice.org.uk/guidance/ng158
Confirmation of PE
Confirmation of the diagnosis requires documentation of a blood clot in a pulmonary artery by an imaging study (such as CTPA). Confirmation of PE with a definitive test is essential because treatment is associated with significant bleeding risk.
[Figure caption and citation for the preceding image starts]: Summary: pulmonary embolism diagnostic pathwayCreated by BMJ Knowledge Centre [Citation ends].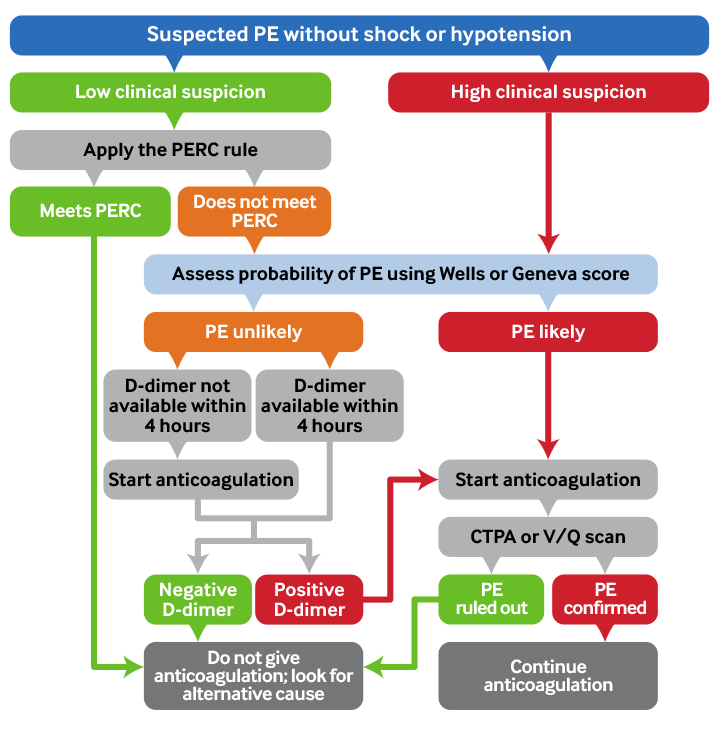
History
History can vary greatly between individuals. Many will report an acute onset of either chest discomfort or dyspnea, but PE may present with more unusual symptoms, or even be asymptomatic.[88]Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011 Feb 8;57(6):700-6.
http://www.onlinejacc.org/content/57/6/700
http://www.ncbi.nlm.nih.gov/pubmed/21292129?tool=bestpractice.com
Pleuritic chest pain and dyspnea are the common presenting features.[88]Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011 Feb 8;57(6):700-6.
http://www.onlinejacc.org/content/57/6/700
http://www.ncbi.nlm.nih.gov/pubmed/21292129?tool=bestpractice.com
[89]Bajaj N, Bozarth AL, Guillot J, et al. Clinical features in patients with pulmonary embolism at a community hospital: analysis of 4 years of data. J Thromb Thrombolysis. 2014 Apr;37(3):287-92.
http://www.ncbi.nlm.nih.gov/pubmed/23681675?tool=bestpractice.com
A sense of apprehension is often reported.[5]Bell WR, Simon TL, DeMets DL. The clinical features of submassive and massive pulmonary emboli. Am J Med. 1977 Mar;62(3):355-60.
http://www.ncbi.nlm.nih.gov/pubmed/842555?tool=bestpractice.com
[90]Stein PD, Willis PW 3rd, DeMets DL. History and physical examination in acute pulmonary embolism in patients without preexisting cardiac or pulmonary disease. Am J Cardiol. 1981 Feb;47(2):218-23.
http://www.ncbi.nlm.nih.gov/pubmed/7468469?tool=bestpractice.com
Hemoptysis and syncope are less common; the latter suggests a larger clot burden, more significant right ventricular dysfunction, and poorer prognosis.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[88]Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011 Feb 8;57(6):700-6.
http://www.onlinejacc.org/content/57/6/700
http://www.ncbi.nlm.nih.gov/pubmed/21292129?tool=bestpractice.com
[89]Bajaj N, Bozarth AL, Guillot J, et al. Clinical features in patients with pulmonary embolism at a community hospital: analysis of 4 years of data. J Thromb Thrombolysis. 2014 Apr;37(3):287-92.
http://www.ncbi.nlm.nih.gov/pubmed/23681675?tool=bestpractice.com
Risk factors for venous thromboembolism (VTE) should be ascertained.
Signs and physical examination
Signs of PE include tachycardia, tachypnea, increased respiratory effort, fever (usually low-grade), and, in more severe cases, hypotension and signs of hypoperfusion (shock).
Physical examination is often nonspecific.[88]Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011 Feb 8;57(6):700-6.
http://www.onlinejacc.org/content/57/6/700
http://www.ncbi.nlm.nih.gov/pubmed/21292129?tool=bestpractice.com
In severe cases, findings of right ventricular overload may be present, such as elevated jugular venous pulsation, loss of palpability of the left ventricular apex (due to posterior displacement of the left ventricle by enlargement of the right ventricle), and a right-sided third heart sound.
PE which has progressed over a period of time may present with physical findings of pulmonary hypertension, such as a right ventricular heave, holosystolic murmur of tricuspid regurgitation, loud pulmonic component of the second heart sound, elevated jugular venous pressure, and pitting edema of the extremities.[91]Courtney DM, Kline JA, Kabrhel C, et al. Clinical features from the history and physical examination that predict the presence or absence of pulmonary embolism in symptomatic emergency department patients: results of a prospective, multicenter study. Ann Emerg Med. 2010 Apr;55(4):307-15.e1.
https://www.doi.org/10.1016/j.annemergmed.2009.11.010
http://www.ncbi.nlm.nih.gov/pubmed/20045580?tool=bestpractice.com
[92]Bajaj R, Ramanakumar A, Mamidala S, et al. Successful treatment of mobile right atrial thrombus and acute pulmonary embolism with intravenous tissue plasminogen activator. BMJ Case Rep. 2013 Jul 25;2013:bcr2013010255.
http://www.ncbi.nlm.nih.gov/pubmed/23892824?tool=bestpractice.com
[93]Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008 Feb;4(1):49-59.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774585
http://www.ncbi.nlm.nih.gov/pubmed/19924277?tool=bestpractice.com
As PE most often originates from lower extremity deep vein thrombosis (DVT), physical findings of this condition may be present.
Patients with suspected PE with shock or hypotension
Shock (end-organ hypoperfusion and a systolic BP <90 mmHg or vasopressor requirement to maintain systolic BP >90 mmHg) or hypotension (systolic BP <90 mmHg or >40mmHg from known baseline for at least 15 minutes) occurs in a minority of cases but portends a high risk of mortality.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
More than 95% of patients who present with acute PE are hemodynamically stable.[94]Laporte S, Mismetti P, Décousus H, et al; RIETE Investigators. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008 Apr 1;117(13):1711-6.
http://circ.ahajournals.org/content/117/13/1711.long
http://www.ncbi.nlm.nih.gov/pubmed/18347212?tool=bestpractice.com
Ideally, PE should be confirmed by CTPA before thrombolytic therapy is administered.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
However, a negative V/Q lung scan effectively excludes PE, and is a radiation- and medium-sparing procedure.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
If the patient is at risk of imminent cardiac arrest, treatment may be commenced on clinical grounds alone.[96]British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003 Jun;58(6):470-83.
http://thorax.bmj.com/content/58/6/470.long
http://www.ncbi.nlm.nih.gov/pubmed/12775856?tool=bestpractice.com
Patients with suspected PE without shock or hypotension
When history and physical exam fail to rule out PE, the pretest probability of PE should be determined using a validated prediction rule and/or clinical judgment.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
A prediction rule may be preferable, particularly for clinicians who rarely evaluate patients for PE, because clinical judgment lacks standardization.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
[97]Lucassen W, Geersing GJ, Erkens PM, et al. Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med. 2011 Oct 4;155(7):448-60.
http://www.ncbi.nlm.nih.gov/pubmed/21969343?tool=bestpractice.com
Assessing clinical probability of PE
Patients with suspected PE can be classified into distinct categories of clinical (pretest) probability that correspond to confirmed PE prevalence, using the original Wells criteria (modified), simplified Wells criteria (modified), original Geneva score (revised), or the simplified Geneva score (revised).[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[98]Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients' probability of pulmonary embolism: increasing the model's utility with the SimpliRED D-dimer. Thromb Haemost. 2000 Mar;83(3):416-20.
http://www.ncbi.nlm.nih.gov/pubmed/10744147?tool=bestpractice.com
[99]Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006 Feb 7;144(3):165-71.
http://www.ncbi.nlm.nih.gov/pubmed/16461960?tool=bestpractice.com
Each of these clinical decision tools assigns a value (a single point, or points) to a series of historic and physical examination features, the sum of which determines whether PE is likely or unlikely.
[
Pulmonary Embolism Wells Score
Opens in new window
]
[
Revised Geneva Score for Estimation of the Clinical Probability of Pulmonary Embolism in Adults
Opens in new window
]
[Figure caption and citation for the preceding image starts]: Original and simplified Wells criteria (modified)Created by the BMJ Knowledge Centre [Citation ends].
[Figure caption and citation for the preceding image starts]: Original and simplified Geneva score (revised)Created by the BMJ Knowledge Centre [Citation ends].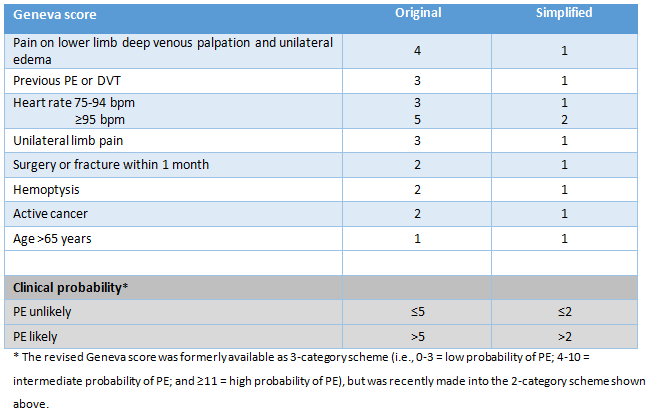
The simplified versions of the modified Wells criteria or revised Geneva score may be preferred in clinical practice because of their ease of use.[100]van Es N, Kraaijpoel N, Klok FA, et al. The original and simplified Wells rules and age-adjusted D-dimer testing to rule out pulmonary embolism: an individual patient data meta-analysis. J Thromb Haemost. 2017 Apr;15(4):678-84.
https://onlinelibrary.wiley.com/doi/full/10.1111/jth.13630
http://www.ncbi.nlm.nih.gov/pubmed/28106338?tool=bestpractice.com
Both simplified versions have been validated; neither has been shown to be superior to the other.[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
[101]Hendriksen JM, Geersing GJ, Lucassen WA, et al. Diagnostic prediction models for suspected pulmonary embolism: systematic review and independent external validation in primary care. BMJ. 2015 Sep 8;351:h4438.
https://www.bmj.com/content/351/bmj.h4438.long
http://www.ncbi.nlm.nih.gov/pubmed/26349907?tool=bestpractice.com
However, the Geneva score is based entirely on objective clinical items and may be more reproducible (the Wells criteria [original and simplified] include the subjective clinical item "alternative diagnosis less likely than PE").[102]Klok FA, Kruisman E, Spaan J, et al. Comparison of the revised Geneva score with the Wells rule for assessing clinical probability of pulmonary embolism. J Thromb Haemost. 2008 Jan;6(1):40-4.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1538-7836.2007.02820.x
http://www.ncbi.nlm.nih.gov/pubmed/17973649?tool=bestpractice.com
The Wells criteria and revised Geneva score categorize patients dichotomously (PE unlikely or PE likely). However, earlier iterations of each tool attributed low, intermediate, or high clinical probabilities of PE. If the two-level classification is used, PE is confirmed in 50% of patients in the PE-likely category compared with 12% in the PE-unlikely category. If the three-level classification is employed, the proportion of patients with confirmed PE will be around 10% in the low probability category, 30% in the intermediate probability category, and 65% in the high probability category.[103]Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010 May;8(5):957-70.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1538-7836.2010.03801.x
http://www.ncbi.nlm.nih.gov/pubmed/20149072?tool=bestpractice.com
PE likely (high clinical probability)
Multiple-detector CTPA should be ordered for patients with a PE likely clinical (pretest) probability.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
V/Q lung scan effectively excludes PE, and is a radiation- and medium-sparing procedure.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
D-dimer testing should not be performed: a normal plasma D-dimer level does not obviate the need for imaging in this patient population.[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
PE unlikely (intermediate or low clinical probability)
Guidelines from the American College of Physicians recommend the application of the PERC to exclude PE in patients initially assessed to have a very low pretest probability of PE.[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
In patients who meet all PERC criteria (age <50 years; initial heart rate <100 bpm; initial oxygen saturation >94% on room air; no unilateral leg swelling; no hemoptysis; no surgery or trauma within the last 4 weeks; no history of VTE; no estrogen use), the risk for PE is considered to be lower than the risk of testing, and no further testing is indicated. Patients who do not meet all of the PERC criteria can be stratified using D-dimer testing.[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
One meta-analysis of studies that assessed the accuracy of PERC to rule out PE reported a sensitivity of 97%.[104]Singh B, Mommer SK, Erwin PJ, et al. Pulmonary embolism rule-out criteria (PERC) in pulmonary embolism - revisited: a systematic review and meta-analysis. Emerg Med J. 2013 Sep;30(9):701-6.
http://emj.bmj.com/content/30/9/701.long
http://www.ncbi.nlm.nih.gov/pubmed/23038695?tool=bestpractice.com
D-dimer test
D-dimer testing is highly sensitive (>95%) but nonspecific.
A normal plasma D-dimer level below threshold safely excludes PE in patients with an unlikely (intermediate or low) pretest probability of PE, and no further testing is required.[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
The risk of PE within 3 months is <1% in these patients.[105]Carrier M, Righini M, Djurabi RK, et al. VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism: a systematic review of management outcome studies. Thromb Haemost. 2009 May;101(5):886-92.
http://www.ncbi.nlm.nih.gov/pubmed/19404542?tool=bestpractice.com
[106]Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010 Jul 15;363(3):266-74.
http://www.ncbi.nlm.nih.gov/pubmed/20592294?tool=bestpractice.com
D-dimer may be adjusted to age (normal is <age × 10 micrograms/L in patients aged ≥50 years) or pre-test probability of disease (1000 nanograms/mL cutoff in low-probability patients, or 500 nanograms/mL in intermediate-probability patients) to increase the specificity, and thus the percentage of patients who can avoid an imaging study.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[107]Kearon C, de Wit K, Parpia S, et al. Diagnosis of pulmonary embolism with d-Dimer adjusted to clinical probability. N Engl J Med. 2019 Nov 28;381(22):2125-34.
https://www.doi.org/10.1056/NEJMoa1909159
http://www.ncbi.nlm.nih.gov/pubmed/31774957?tool=bestpractice.com
[108]Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014 Mar 19;311(11):1117-24.
https://www.doi.org/10.1001/jama.2014.2135
http://www.ncbi.nlm.nih.gov/pubmed/24643601?tool=bestpractice.com
In patients with cancer, using an age-adjusted D-dimer cut-off doubled the proportion of patients in whom PE could be excluded by clinical decision rule and D-dimer, without imaging.[109]Wilts IT, Le Gal G, Den Exter PL, et al. Performance of the age-adjusted cut-off for D-dimer in patients with cancer and suspected pulmonary embolism. Thromb Res. 2017 Apr;152:49-51.
http://www.ncbi.nlm.nih.gov/pubmed/28226257?tool=bestpractice.com
The YEARS algorithm with risk-adapted D-dimer thresholds has been studied in pregnant patients suspected of PE.[110]van der Pol LM, Tromeur C, Bistervels IM, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019 Mar 21;380(12):1139-49.
https://www.doi.org/10.1056/NEJMoa1813865
http://www.ncbi.nlm.nih.gov/pubmed/30893534?tool=bestpractice.com
Patients with an abnormal D-dimer level should undergo multiple-detector CTPA (or V/Q lung scan if CTPA is contraindicated) to confirm or exclude a diagnosis of PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[87]Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015 Nov 3;163(9):701-11.
https://www.acpjournals.org/doi/10.7326/M14-1772
http://www.ncbi.nlm.nih.gov/pubmed/26414967?tool=bestpractice.com
[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
Initial imaging studies
CTPA confirms the diagnosis by direct visualization of thrombus in a pulmonary artery, where it appears as a partial or complete intraluminal filling defect. The likelihood ratio to rule in a PE with a filling defect in the segmental or subsegmental branches is 24.1 (range of 12.4 to 46.7), whereas the likelihood to rule it out is 0.11 (range of 0.06 to 0.19), which means that CTPA has the best diagnostic accuracy of all advanced noninvasive imaging methods.[111]Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005 Apr 28;352(17):1760-8.
https://www.nejm.org/doi/10.1056/NEJMoa042905
http://www.ncbi.nlm.nih.gov/pubmed/15858185?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Contrasted CTPA scan showing subsegmental right pulmonary artery emboli (see arrows)From the collection of Seth W. Clemens; used with permission [Citation ends].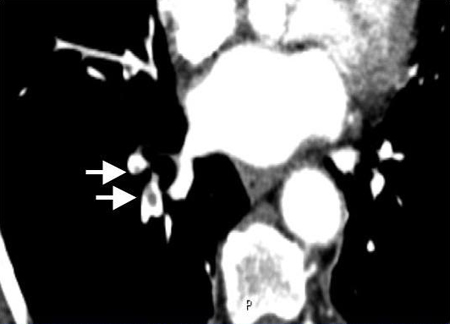
V/Q lung scan, preferably using single photon emission computed tomography (SPECT, which may reduce the number of inconclusive scans), is an alternative to CTPA.[112]Phillips JJ, Straiton J, Staff RT. Planar and SPECT ventilation/perfusion imaging and computed tomography for the diagnosis of pulmonary embolism: a systematic review and meta-analysis of the literature, and cost and dose comparison. Eur J Radiol. 2015 Jul;84(7):1392-400.
http://www.ncbi.nlm.nih.gov/pubmed/25868674?tool=bestpractice.com
A negative V/Q scan effectively excludes PE. V/Q scan is a radiation- and medium-sparing procedure and may be appropriate for patients with contraindications or relative contraindications to CT (e.g., contrast allergy, moderate to severe renal failure, pregnancy, young patients).[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Subsegmental PE
CTPA appears to increase the proportion of patients diagnosed with subsegmental PE without lowering the 3-month risk of thromboembolism, leading to potential over-diagnosis (because subsegmental PE may not warrant anticoagulant treatment in select patients).[113]Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010 Aug;8(8):1716-22.
https://onlinelibrary.wiley.com/doi/full/10.1111/j.1538-7836.2010.03938.x
http://www.ncbi.nlm.nih.gov/pubmed/20546118?tool=bestpractice.com
The risk of over-diagnosis may be mitigated by adherence to diagnostic algorithms that limit imaging in patients at lower probability of disease.[114]Adams DM, Stevens SM, Woller SC, et al. Adherence to PIOPED II investigators' recommendations for computed tomography pulmonary angiography. Am J Med. 2013 Jan;126(1):36-42.
https://www.doi.org/10.1016/j.amjmed.2012.05.028
http://www.ncbi.nlm.nih.gov/pubmed/23177546?tool=bestpractice.com
Other imaging studies
A normal chest x-ray does not eliminate PE as a diagnosis.[115]American College of Radiology. ACR Appropriateness Criteria®: suspected pulmonary embolism. 2022 [internet publication].
https://acsearch.acr.org/docs/69404/Narrative
However, a chest-ray may rule out other causes of a patient’s symptoms such as pneumothorax or pneumonia.[115]American College of Radiology. ACR Appropriateness Criteria®: suspected pulmonary embolism. 2022 [internet publication].
https://acsearch.acr.org/docs/69404/Narrative
Magnetic resonance angiography can be used to evaluate the central and segmental arteries.[115]American College of Radiology. ACR Appropriateness Criteria®: suspected pulmonary embolism. 2022 [internet publication].
https://acsearch.acr.org/docs/69404/Narrative
Three different techniques are available: gadolinium-contrast enhanced angiography (Gd-MRA), real-time angiography (RT-MRA), and MR-perfusion images.[116]Clemens S. Newer modalities for detection of pulmonary emboli. Am J Med. 2007 Oct;120(10 suppl 2):S2-12.
http://www.ncbi.nlm.nih.gov/pubmed/17916456?tool=bestpractice.com
Transthoracic echocardiography is generally not indicated in the diagnosis of acute PE, but it is useful in identifying right ventricular strain and assisting with severity classification and determining prognosis.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[115]American College of Radiology. ACR Appropriateness Criteria®: suspected pulmonary embolism. 2022 [internet publication].
https://acsearch.acr.org/docs/69404/Narrative
Echocardiographic evidence of right heart thrombi is significantly associated with increased 30-day mortality in patients diagnosed with acute PE.[117]Barrios D, Rosa-Salazar V, Morillo R, et al. Prognostic significance of right heart thrombi in patients with acute symptomatic pulmonary embolism: systematic review and meta-analysis. Chest. 2017 Feb;151(2):409-16.
http://www.ncbi.nlm.nih.gov/pubmed/27746202?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Gd-MRA showing a right main pulmonary artery pulmonary embolism (see arrow)From the collection of Seth W. Clemens; used with permission [Citation ends].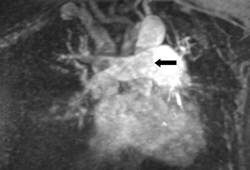
Despite its diagnostic accuracy, pulmonary angiography is rarely used for the diagnosis or exclusion of PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[118]Kearon C. Diagnosis of suspected venous thromboembolism. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;2016(1):397-403.
http://asheducationbook.hematologylibrary.org/content/2016/1/397.long
http://www.ncbi.nlm.nih.gov/pubmed/27913507?tool=bestpractice.com
It is associated with risk of morbidity/mortality, and (less invasive) CTPA affords comparable diagnostic precision.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[119]Stein PD, Athanasoulis C, Alavi A, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation. 1992 Feb;85(2):462-8.
http://www.ncbi.nlm.nih.gov/pubmed/1735144?tool=bestpractice.com
Laboratory investigations
Baseline laboratory tests including prothrombin time (PT), activated partial thromboplastin time (aPTT), and international normalized ratio (INR) are important to aid decisions about the safety and type of initial anticoagulation selected. Renal and hepatic function panels also help determine the appropriate choice of anticoagulant therapy, as different agents carry precautions or are contraindicated in renal or hepatic dysfunction.[120]Ansell JE. Management of venous thromboembolism: clinical guidance from the Anticoagulation Forum. J Thromb Thrombolysis. 2016 Jan;41(1):1-2.
https://www.doi.org/10.1007/s11239-015-1320-5
http://www.ncbi.nlm.nih.gov/pubmed/26780735?tool=bestpractice.com
Complete blood count may detect hematologic abnormalities.
Troponin can be used to assist in determining the severity category of acute PE, which impacts management decisions. It is suggested in patients who have either an elevated Pulmonary Embolism Severity Index (PESI) category, or abnormalities of the right ventricle on imaging.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
Thrombophilia screen
Thrombophilia commonly refers to five hereditary conditions (Factor V Leiden, prothrombin gene 20210A, deficiencies in antithrombin, protein C deficiency, and protein S deficiency) and antiphospholipid syndrome (an acquired condition). However, many gene variants and acquired conditions modify thrombosis risk.[121]Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016 Jan;41(1):154-64.
https://link.springer.com/article/10.1007/s11239-015-1316-1
http://www.ncbi.nlm.nih.gov/pubmed/26780744?tool=bestpractice.com
Indications for screening are controversial.[121]Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016 Jan;41(1):154-64.
https://link.springer.com/article/10.1007/s11239-015-1316-1
http://www.ncbi.nlm.nih.gov/pubmed/26780744?tool=bestpractice.com
[122]Connors JM. Thrombophilia testing and venous thrombosis. N Engl J Med. 2017 Sep 21;377(12):1177-87.
https://www.doi.org/10.1056/NEJMra1700365
http://www.ncbi.nlm.nih.gov/pubmed/28930509?tool=bestpractice.com
Hereditary thrombophilia does not sufficiently modify the predicted risk of recurrent thrombosis to affect treatment decisions, and a conservative approach to testing is reasonable.[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
If a thrombophilia screen is indicated, it should be deferred until a minimum of 3 months of anticoagulant therapy has been completed because some thrombophilia tests are influenced by the presence of acute thrombosis or anticoagulant therapy.[121]Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016 Jan;41(1):154-64.
https://link.springer.com/article/10.1007/s11239-015-1316-1
http://www.ncbi.nlm.nih.gov/pubmed/26780744?tool=bestpractice.com
Some guidelines suggest testing only in situations where the result is likely to change a clinical decision (such as in patients with unprovoked DVT or PE who are considering stopping anticoagulants).[22]National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Aug 2023 [internet publication].
https://www.nice.org.uk/guidance/ng158
If a hereditary thrombophilia screen is considered, it should be deferred until a minimum of 3 months of anticoagulant therapy has been completed because some thrombophilia tests are influenced by the presence of acute thrombosis or anticoagulant therapy.[121]Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016 Jan;41(1):154-64.
https://link.springer.com/article/10.1007/s11239-015-1316-1
http://www.ncbi.nlm.nih.gov/pubmed/26780744?tool=bestpractice.com
The presence of a hereditary thrombophilia does not significantly increase the predicted risk of recurrent VTE after a provoked DVT, and guidelines discourage testing in this setting.[123]Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely®campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14.
https://www.doi.org/10.1182/asheducation-2013.1.9
http://www.ncbi.nlm.nih.gov/pubmed/24319155?tool=bestpractice.com
Antiphospholipid syndrome
Antiphospholipid antibodies may predict a higher risk of future thrombosis following an initial VTE event and may impact selection of therapy.[59]Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018 May 24;378(21):2010-21.
http://www.ncbi.nlm.nih.gov/pubmed/29791828?tool=bestpractice.com
Controversy exists regarding whether broad screening for antiphospholipid antibodies or screening only on the basis of clinical suspicion should be preferred.[124]Fazili M, Stevens SM, Woller SC. Direct oral anticoagulants in antiphospholipid syndrome with venous thromboembolism: impact of the European Medicines Agency guidance. Res Pract Thromb Haemost. 2020 Jan;4(1):9-12.
https://www.doi.org/10.1002/rth2.12287
http://www.ncbi.nlm.nih.gov/pubmed/31989078?tool=bestpractice.com
[125]European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC). PRAC recommendations on signals. Jun 2019 [internet publication].
https://www.ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-13-16-may-2019-prac-meeting_en.pdf
Some guidelines suggest testing only in situations where the result is likely to change a clinical decision (such as in patients with unprovoked DVT or PE who are considering stopping anticoagulants, however these guidelines recommend seeking specialist advice as these tests may be affected by anticoagulants).[22]National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Aug 2023 [internet publication].
https://www.nice.org.uk/guidance/ng158
For antiphospholipid antibody screening, cardiolipin and beta-2 glycoprotein-I antibodies can be performed without regard to the presence of anticoagulants; however, most anticoagulants interfere with assays for lupus anticoagulant.[59]Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018 May 24;378(21):2010-21.
http://www.ncbi.nlm.nih.gov/pubmed/29791828?tool=bestpractice.com
Arterial blood gas analysis is of limited utility
Hypoxemia is considered to be a typical finding in acute PE, but arterial blood gas analysis is of very limited diagnostic utility, alone or in combination with other clinical variables, in suspected PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[126]Rodger MA, Carrier M, Jones GN, et al. Diagnostic value of arterial blood gas measurement in suspected pulmonary embolism. Am J Respir Crit Care Med. 2000 Dec;162(6):2105-8.
https://www.atsjournals.org/doi/full/10.1164/ajrccm.162.6.2004204
http://www.ncbi.nlm.nih.gov/pubmed/11112122?tool=bestpractice.com
A PaO₂ <80 mmHg, a PaCO₂ <36 mmHg, or an abnormal alveolar-arterial gradient (A–aO₂) are not predictive of PE in patients suspected of having PE.[126]Rodger MA, Carrier M, Jones GN, et al. Diagnostic value of arterial blood gas measurement in suspected pulmonary embolism. Am J Respir Crit Care Med. 2000 Dec;162(6):2105-8.
https://www.atsjournals.org/doi/full/10.1164/ajrccm.162.6.2004204
http://www.ncbi.nlm.nih.gov/pubmed/11112122?tool=bestpractice.com
In patients with suspected acute PE with normal arterial blood gas results, PE could not be excluded in 38% of those without cardiopulmonary disease and 14% with pre-existing cardiopulmonary disease, respectively.[127]Stein PD, Goldhaber SZ, Henry JW, et al. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest. 1996 Jan;109(1):78-81.
http://www.ncbi.nlm.nih.gov/pubmed/8549223?tool=bestpractice.com
Other investigations
Electrocardiography (ECG) cannot definitively establish or eliminate PE as a diagnosis, and specific findings may only be suggestive of PE.[89]Bajaj N, Bozarth AL, Guillot J, et al. Clinical features in patients with pulmonary embolism at a community hospital: analysis of 4 years of data. J Thromb Thrombolysis. 2014 Apr;37(3):287-92.
http://www.ncbi.nlm.nih.gov/pubmed/23681675?tool=bestpractice.com
[128]Brown G, Hogg K. Best evidence topic report: diagnostic utility of electrocardiogram for diagnosing pulmonary embolism. Emerg Med J. 2005 Oct;22(10):729-30.
http://emj.bmj.com/content/22/10/729.2.long
http://www.ncbi.nlm.nih.gov/pubmed/16189038?tool=bestpractice.com
[129]Sukhija R, Aronow WS, Ahn C, et al. Electrocardiographic abnormalities in patients with right ventricular dilation due to acute pulmonary embolism. Cardiology. 2006;105(1):57-60.
http://www.ncbi.nlm.nih.gov/pubmed/16254425?tool=bestpractice.com
ECG can, however, be used to assess right ventricular function in patients with confirmed PE without shock or hypotension.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[21]Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. 2021 Dec;160(6):e545-608.
https://journal.chestnet.org/article/S0012-3692(21)01506-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34352278?tool=bestpractice.com
Right ventricular dysfunction is predictive of adverse outcome and enables risk stratification in these patients.[130]Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med. 2009 Mar;122(3):257-64.
http://www.ncbi.nlm.nih.gov/pubmed/19272487?tool=bestpractice.com
[131]Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011 Apr 26;123(16):1788-830.
http://circ.ahajournals.org/content/123/16/1788.long
http://www.ncbi.nlm.nih.gov/pubmed/21422387?tool=bestpractice.com
[132]Weekes AJ, Thacker G, Troha D, et al. Diagnostic accuracy of right ventricular dysfunction markers in normotensive emergency department patients with acute pulmonary embolism. Ann Emerg Med. 2016 Sep;68(3):277-91.
http://www.ncbi.nlm.nih.gov/pubmed/26973178?tool=bestpractice.com
If a definitive imaging modality is unavailable, echocardiography may be considered for patients with suspected PE presenting with shock or hypotension.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
Special patient populations
Symptoms and signs of VTE may be less specific in pregnant women than in nonpregnant patients.[95]Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016 Mar 1;67(8):976-90.
http://www.onlinejacc.org/content/67/8/976
http://www.ncbi.nlm.nih.gov/pubmed/26916489?tool=bestpractice.com
D-dimer levels increase through normal pregnancy, complicating its use as a test to exclude suspected PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
However, increasing evidence supports the use of pre-test probability algorithms adapted to pregnant patients with D-dimer.[110]van der Pol LM, Tromeur C, Bistervels IM, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019 Mar 21;380(12):1139-49.
https://www.doi.org/10.1056/NEJMoa1813865
http://www.ncbi.nlm.nih.gov/pubmed/30893534?tool=bestpractice.com
[133]Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med. 2018 Oct 23;169(11):766-73.
https://www.doi.org/10.7326/M18-1670
http://www.ncbi.nlm.nih.gov/pubmed/30357273?tool=bestpractice.com
Guidelines support use of multi-step algorithms over universal imaging in pregnant patients with suspected PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
When an algorithm indicates that imaging is indicated in a pregnant patient suspected of PE, exposure to radiation-associated imaging should be minimized. Bilateral venous compression ultrasound to establish the presence of thrombosis suggestive of PE is recommended in pregnant patients with suspected PE.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
[134]Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Respir Crit Care Med. 2011 Nov 15;184(10):1200-8.
https://www.atsjournals.org/doi/full/10.1164/rccm.201108-1575ST#.UnJdTVNZit8
http://www.ncbi.nlm.nih.gov/pubmed/22086989?tool=bestpractice.com
Chest x-ray is the first radiation-associated procedure if PE is suspected.[134]Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Respir Crit Care Med. 2011 Nov 15;184(10):1200-8.
https://www.atsjournals.org/doi/full/10.1164/rccm.201108-1575ST#.UnJdTVNZit8
http://www.ncbi.nlm.nih.gov/pubmed/22086989?tool=bestpractice.com
In the setting of a normal chest x-ray, American Thoracic Society consensus guidelines recommend lung scintigraphy (with V/Q scan).[134]Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Respir Crit Care Med. 2011 Nov 15;184(10):1200-8.
https://www.atsjournals.org/doi/full/10.1164/rccm.201108-1575ST#.UnJdTVNZit8
http://www.ncbi.nlm.nih.gov/pubmed/22086989?tool=bestpractice.com
Pregnant women with a nondiagnostic V/Q scan, in whom further investigation is deemed appropriate, may undergo CTPA.[134]Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Respir Crit Care Med. 2011 Nov 15;184(10):1200-8.
https://www.atsjournals.org/doi/full/10.1164/rccm.201108-1575ST#.UnJdTVNZit8
http://www.ncbi.nlm.nih.gov/pubmed/22086989?tool=bestpractice.com
European Society of Cardiology guidelines suggest that CTPA in patients with abnormal chest x-ray and either V/Q scan or CTPA if chest x-ray is normal.[4]Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan 21;41(4):543-603.
https://academic.oup.com/eurheartj/article/41/4/543/5556136
http://www.ncbi.nlm.nih.gov/pubmed/31504429?tool=bestpractice.com
The administered dose of radiopharmaceutical should be reduced by a factor of 2 when lung scans are indicated in pregnant women; longer acquisition times should be used to achieve adequate imaging.[112]Phillips JJ, Straiton J, Staff RT. Planar and SPECT ventilation/perfusion imaging and computed tomography for the diagnosis of pulmonary embolism: a systematic review and meta-analysis of the literature, and cost and dose comparison. Eur J Radiol. 2015 Jul;84(7):1392-400.
http://www.ncbi.nlm.nih.gov/pubmed/25868674?tool=bestpractice.com
Adolescents and young adults
CTPA should be used with discretion, especially if PE can be ruled out by other noninvasive methods with less radiation exposure.[135]Arnold RW, Janitz E, Poulton TB, et al. Pulmonary CT angiography to evaluate for pulmonary embolism in children visiting adult-centered community hospitals. AJR Am J Roentgenol. 2011 Jun;196(6):W823-30.
https://www.ajronline.org/doi/full/10.2214/AJR.10.5951
http://www.ncbi.nlm.nih.gov/pubmed/21606276?tool=bestpractice.com




