Approach
The main aims of treatment include optimization of height, induction and maintenance of pubertal development, treatment of ongoing ovarian hormone deficiency, and screening for and treatment of comorbidities or complications.[30]
Poor growth
Short stature and poor growth are often the primary symptoms.
Recombinant human growth hormone (GH)
Often considered to enhance adult height. The goal is to help girls achieve a height sufficient to prevent disability and enable them to function independently (e.g., drive a car) and to promote social integration.
Treatment should be begun from the time girls drop off the normal growth curve until growth velocity is less than 2 cm/year.[30]
Treatment of very young girls prior to growth failure prevents the development of short stature. Near adult height standard deviation score was >- 2.0 for 76% of girls starting growth hormone on average at 24 months and 60% for girls starting growth hormone at 4 years.[31]
Treatment effect is monitored by the growth response and insulin-like growth factor 1 (IGF-1) levels, which should be maintained below the upper limit of normal.
Daily GH treatment for 5 years started at age 8 years yields a 7 cm gain in height in girls with Turner syndrome compared with those not receiving GH.[32]
Daily GH treatment for 8.3 to 8.9 years started at age 6.5 to 6.9 years yields an 11.9 to 15.7 cm gain in height in girls with Turner syndrome treated with two different doses of growth hormone.[33]
Failure of a good growth response is commonly due to hypothyroidism, celiac disease, or noncompliance.
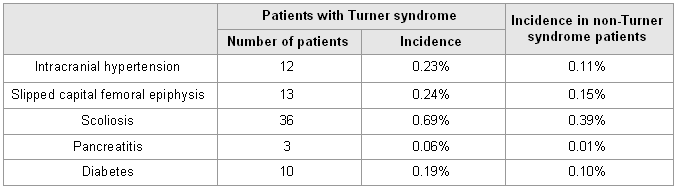
Adverse effects over a 4- to 5-year follow-up include increased intracranial pressure, slipped capital femoral epiphyses, scoliosis, pancreatitis, and, possibly, an increased onset of type 1 diabetes.[3][34][Figure caption and citation for the preceding image starts]: Incidence of adverse effects of GH treatmentData from the National Cooperative Growth Study (Bolar K, et al. J Clin Endocrinol Metab. 2008;93:344-351) [Citation ends].
Oxandrolone
The major impediment to successful GH treatment is late diagnosis, which is not uncommon.
For girls diagnosed very late with only a small time window for treatment, or for those who cannot obtain GH, some pediatricians add oxandrolone, a nonaromatizable oral androgen, to GH treatment to promote linear growth.[35] Oxandrolone treatment usually promotes a few centimeters of additional height.[36][37][38] However, it can, even at low doses, inhibit breast development and cause virilization due to its androgenic effects.[36][37]
Puberty delay
The aim is to induce pubertal development on a par with peers and as physiologically as possible. This also ensures that the girls experience the salutary effects of estrogen on bone and other tissues, and undergo optimal psychosocial adjustment to their condition.
Treatment includes gradually increasing doses of estrogen therapy, and then estrogen combined with cyclic progesterone treatment. Treatment with combination agents (conjugated equine estrogen and medroxyprogesterone) developed for menopausal women is not recommended. Puberty should not be induced using contraceptive pills because the doses of estrogen are too high and the androgenic progestins impair optimal breast development.
Estrogen replacement
If no spontaneous breast development has occurred by the age of 11- 12 years and serum follicle-stimulating hormone is elevated, estrogen replacement therapy should be started.
The preferred preparation is transdermal estradiol in the smallest available dose.[3] Transdermal delivery avoids first-pass effects on the liver. Oral estradiol may also be used as an alternative.
If potential for linear growth remains (patient may be already on GH treatment), low dose with a slower increase in dose should be continued until an optimum height is achieved. Higher doses bring growth to an end. The bone age should be monitored and, if found to be advancing rapidly, the dosage should be reduced.
If there is no potential for further growth or if there is no evidence of a rapidly advancing bone age, the dose of estradiol is increased gradually, over approximately 2 years, to a full adult dose or until vaginal bleeding occurs.
Cyclic progesterone
Is added to estrogen therapy once there is breakthrough bleeding or after 2 years of estrogen treatment.[3]
Oral micronized progesterone is recommended for the last 10 days of each month to induce menstruation. Treatment for the last 2 weeks of a 3-month cycle is also an option.
Congenital cardiac anomalies
Aortic coarctation and bicuspid aortic valves are the most common abnormalities. Other cardiac abnormalities include partial anomalous pulmonary veins, left heart hypoplasia, and a dilated aorta. Prevalence of cardiovascular defects is much higher in those with clear evidence of fetal lymphedema, which can be described as swelling of tissues, especially of the head and neck, due to impaired lymphatic development. The common postnatal manifestations are neck webbing and low-set ears and hairline.
At the time of initial diagnosis, no matter what age, a comprehensive cardiovascular evaluation needs to be done by a specialist in congenital heart disease and appropriate treatment started.[29] A functionally bicuspid valve as a result of complete or partial fusion of the right and left coronary leaflets is seen in 30% of asymptomatic patients, and poses a risk for infection, valve deterioration, and aortic dilation and dissection.[16]
Congenital cardiovascular defects constitute the major source of premature mortality in Turner syndrome. Children with cardiac abnormalities should be transferred later to an adult congenital heart disease clinic in view of clear ongoing morbidity and mortality associated with the defects.
Ongoing management after establishment of cyclical bleeding
Ovarian HRT
Oral or transdermal estrogen therapy should be used for hypoestrogenism, to reduce osteoporosis, cardiovascular disease, urogenital atrophy, and improve quality of life.[39]
Women with Turner syndrome should normally continue on HRT after induction and development of puberty. HRT should continue until about 50 years of age, after which further estrogen therapy may be given depending on individuals' risk/benefit considerations.[40]
Estrogen should be combined with progestogen therapy (administered continuously or cyclically) to prevent endometrial hyperplasia and cancer.[39]
For women seeking to prevent pregnancy, combined hormonal contraceptives are more effective than estrogen hormone therapy. A levonorgestrel intrauterine device is another acceptable option.[39]
Low bone mass should be managed with estrogen replacement hormone therapy, adequate calcium in diet, normal vitamin D levels, and weight-bearing activities, not bisphosphonates.[39]
Risk of not using ovarian HRT for young women with premature ovarian failure includes severe premature osteoporosis.[Figure caption and citation for the preceding image starts]: Importance of estrogen therapy in young adults with Turner syndrome (TS): X-ray figure A shows near collapse of T11, diffuse osteoporosis, and dorsal kyphosis in a woman with TS who discontinued HRT at age 18 years. Figure B shows normal spinal architecture and bone health in another woman with TS, age 30 years, who has taken HRT consistently since age 12.8 yearsFrom the personal collection of Carolyn Bondy, MS, MD (NIH study) [Citation ends].

Breast implants
Girls who have prominent signs of fetal lymphedema and hypoplastic nipples may not achieve adequate breast development with estrogen treatment and so may require breast implants.
Fertility and pregnancy
Most women with Turner syndrome are infertile, but spontaneous pregnancy may occur in approximately 5% to 8%.[3] There is increased incidence of a fetus with aneuploidy when women with TS use their own oocytes. Pregnancy exacerbates underlying metabolic, hypertensive, and cardiovascular problems and may be catastrophic for women with Turner syndrome; therefore, girls over the age of 10 years need close monitoring with regard to potential fertility and education on reproductive issues and sexual behaviors.
Given that fertility in women with TS declines rapidly with age, offering fertility treatment at a young age should be considered.[3] Young women with mosaic TS should be counseled about the possibility of controlled ovarian hyperstimulation and oocyte cryopreservation, but routine oocyte retrieval is not recommended in girls aged <12 years.[3] Women with Turner syndrome may be interested in pregnancy via assisted reproduction using donor oocytes. Such pregnancies may have an unusually high risk for maternal death from aortic dissection or rupture, and from pre-eclampsia and its complications.[41][42]
Very stringent prepregnancy screening and patient education as to risks are imperative prior to attempting assisted reproduction for women with Turner syndrome.[43] The American Heart Association and International Turner Syndrome Consensus Group have made recommendations for management of pregnant women with TS; this should be undertaken by a multidisciplinary team, including a maternal-fetal medicine specialist and cardiologist with appropriate expertise.[3][29]
Use of this content is subject to our disclaimer