Rapid evaluation and diagnosis is the cornerstone of successful therapy for ischemic stroke. Prioritize stabilizing the patient by managing any airway, breathing, and circulatory insufficiencies requiring urgent treatment; continue to give supportive care as needed (see section on "Supportive care"). The goals of treatment are to:
Restore blood flow
Support energy metabolism in ischemic tissue
Treat complications of stroke-related edema
Prevent common acute medical complications.
Intravenous thrombolysis
See section on "Candidates for intravenous thrombolysis with alteplase" for recommendations on which patients are eligible for treatment with intravenous thrombolysis.
There are three intravenous formulations of recombinant tissue plasminogen activator (r-tPA) available in the US: alteplase, tenecteplase, and reteplase. Only alteplase is currently approved for use in acute ischemic stroke. The other two are approved for use in acute myocardial infarction only.
Alteplase promotes thrombolysis and thereby recanalization and reperfusion. Early administration of intravenous alteplase is recommended for appropriate patients who meet the defined criteria for thrombolysis.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Early initiation of intravenous thrombolysis (i.e., within 4.5 hours of onset of symptoms, if it is not contraindicated) is associated with improved functional outcomes.[145]Wardlaw JM, Murray V, Berge E, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014 Jul 29;(7):CD000213.
https://www.doi.org/10.1002/14651858.CD000213.pub3
http://www.ncbi.nlm.nih.gov/pubmed/25072528?tool=bestpractice.com
[146]Man S, Xian Y, Holmes DN, et al. Association between thrombolytic door-to-needle time and 1-year mortality and readmission in patients with acute ischemic stroke. JAMA. 2020 Jun 2;323(21):2170-84.
https://www.doi.org/10.1001/jama.2020.5697
http://www.ncbi.nlm.nih.gov/pubmed/32484532?tool=bestpractice.com
[147]Alamowitch S, Turc G, Palaiodimou L, et al. European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke. Eur Stroke J. 2023 Mar;8(1):8-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10069183
http://www.ncbi.nlm.nih.gov/pubmed/37021186?tool=bestpractice.com
In a retrospective cohort study of more than 61,000 patients ages 65 years or older with acute ischemic stroke, shorter door-to-needle times were associated with lower all-cause mortality and lower all-cause readmission at 1 year.[146]Man S, Xian Y, Holmes DN, et al. Association between thrombolytic door-to-needle time and 1-year mortality and readmission in patients with acute ischemic stroke. JAMA. 2020 Jun 2;323(21):2170-84.
https://www.doi.org/10.1001/jama.2020.5697
http://www.ncbi.nlm.nih.gov/pubmed/32484532?tool=bestpractice.com
Administration of alteplase should not be delayed by additional tests unless a specific contraindication is suspected and must be ruled out. Blood glucose should be normalized before initiating alteplase treatment.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Orolingual edema is a rare but potentially serious complication.
In the frequent situation where the onset of symptoms was not witnessed, the time of onset must be presumed to be the time at which the patient was last witnessed to be well.
Trials of alteplase for thrombolysis in patients with acute ischemic stroke and no contraindications suggest that the ideal window of opportunity for treatment is up to 4.5 hours after the onset of neurologic symptoms.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[148]Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317-29.
https://www.nejm.org/doi/10.1056/NEJMoa0804656
http://www.ncbi.nlm.nih.gov/pubmed/18815396?tool=bestpractice.com
For patients with severe acute stroke, goal time from emergency department arrival to initiation of intravenous alteplase (if indicated) is 60 minutes.[117]Alberts MJ, Latchaw RE, Jagoda A, et al. Revised and updated recommendations for the establishment of primary stroke centers: a summary statement from the brain attack coalition. Stroke. 2011 Sep;42(9):2651-65.
https://www.ahajournals.org/doi/full/10.1161/strokeaha.111.615336
http://www.ncbi.nlm.nih.gov/pubmed/21868727?tool=bestpractice.com
[149]Whiteley WN, Emberson J, Lees KR, et al. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016 Aug;15(9):925-33.
http://www.ncbi.nlm.nih.gov/pubmed/27289487?tool=bestpractice.com
Alteplase is approved in the US for use within 3 hours of onset of stroke symptoms, and in Europe for use within 4.5 hours.
Tenecteplase is at least as effective and safe as alteplase.[147]Alamowitch S, Turc G, Palaiodimou L, et al. European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke. Eur Stroke J. 2023 Mar;8(1):8-54.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10069183
http://www.ncbi.nlm.nih.gov/pubmed/37021186?tool=bestpractice.com
[150]Kheiri B, Osman M, Abdalla A, et al. Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. J Thromb Thrombolysis. 2018 Nov;46(4):440-50.
http://www.ncbi.nlm.nih.gov/pubmed/30117036?tool=bestpractice.com
[151]Burgos AM, Saver JL. Evidence that tenecteplase Is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019 Aug;50(8):2156-62.
https://www.doi.org/10.1161/STROKEAHA.119.025080
http://www.ncbi.nlm.nih.gov/pubmed/31318627?tool=bestpractice.com
[152]American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Acute Ischemic Stroke, Lo BM, Carpenter CR, et al. Clinical policy: critical issues in the management of adult patients presenting to the emergency department with acute ischemic stroke. Ann Emerg Med. 2023 Aug;82(2):e17-e64.
https://www.acep.org/patient-care/clinical-policies/acute-ischemic-stroke
http://www.ncbi.nlm.nih.gov/pubmed/37479410?tool=bestpractice.com
The American Heart Association/American Stroke Association (AHA/ASA) recommend to consider tenecteplase as an alternative to alteplase in patients with minor neurologic impairment and no major intracranial occlusion.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Information on the benefits and risks of intravenous thrombolysis should be given to the patient, if competent, or to a surrogate decision-maker, if present. Verbal or written consent should be obtained if feasible. In the frequent situation where the patient is not competent to make medical decisions, and family or a surrogate decision-maker cannot be identified or approached in a timely manner, it is justifiable to proceed with alteplase in an otherwise eligible adult patient with a disabling ischemic stroke.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
If a patient lacks decisional capacity, does not have a determinative advance directive (one that offers guidance in this usually unanticipated situation), and no authorized surrogate is available, interventions may be provided based on the ethical and common law presumption of consent; that is, the rationale that reasonable people would consent to treatment if they could be asked. The imminent risk of significant disability also justifies emergent treatment in these circumstances.[153]Sattin JA, Chiong W, Bonnie RJ, et al. Consent issues in the management of acute ischemic stroke: AAN position statement. Neurology. 2022 Jan 11;98(2):73-9.
https://www.doi.org/10.1212/WNL.0000000000013040
http://www.ncbi.nlm.nih.gov/pubmed/35312627?tool=bestpractice.com
Decision-makers should be informed that r-tPA treatment is associated with a better outcome in around 1 in 3 people treated, and with a worse outcome in around 3 in 100 people treated.[154]Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol. 2004 Jul;61(7):1066-70.
http://jamanetwork.com/journals/jamaneurology/fullarticle/786159
http://www.ncbi.nlm.nih.gov/pubmed/15262737?tool=bestpractice.com
Overall, 1 in 8 people treated with r-tPA have a complete or near-complete recovery who otherwise would have been disabled.[155]National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995 Dec 14;333(24):1581-7.
https://www.nejm.org/doi/full/10.1056/NEJM199512143332401
http://www.ncbi.nlm.nih.gov/pubmed/7477192?tool=bestpractice.com
The absence of definitive evidence on the efficacy of thrombolysis and endovascular therapy in patients with premorbid disability or dementia results in difficult decisions about the use of these therapies. A pragmatic case-by-case approach is recommended in these patients.[156]Ganesh A, Fraser JF, Gordon Perue GL, et al. Endovascular treatment and thrombolysis for acute ischemic stroke in patients with premorbid disability or dementia: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2022 May;53(5):e204-e217.
https://www.doi.org/10.1161/STR.0000000000000406
http://www.ncbi.nlm.nih.gov/pubmed/35343235?tool=bestpractice.com
Contraindications to intravenous thrombolysis with alteplase
The following are contraindications to alteplase treatment from the AHA/ASA guidelines:[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Absolute contraindications:
Onset of symptoms >9 hours[157]Ma H, Campbell BC, Parsons MW, et al; EXTEND Investigators. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019 May 9;380(19):1795-803.
https://www.nejm.org/doi/full/10.1056/NEJMoa1813046
http://www.ncbi.nlm.nih.gov/pubmed/31067369?tool=bestpractice.com
CT reveals acute intracranial hemorrhage
History of severe head trauma
Symptoms suggestive of subarachnoid hemorrhage
Patients with platelets <100,000/mm³, international normalized ratio (INR) >1.7, activated PTT (aPTT) >40 seconds, or prothrombin time >15 seconds
Patient has received a dose of low-molecular-weight heparin within the previous 24 hours
Evidence of active bleeding on examination
Symptoms consistent with infective endocarditis
Known or suspected association between the acute ischemic stroke and aortic arch dissection
Relative contraindications:
History of prior stroke in the previous 3 months
History of previous intracranial hemorrhage
History of intracranial/intraspinal surgery within 3 months
Patient is taking antiplatelet agents that inhibit the glycoprotein IIb/IIIa receptor
History of an intra-axial intracranial neoplasm
History of gastrointestinal malignancy or recent bleeding event in the previous 21 days
History of major surgery or serious trauma in the previous 14 days
Patient is taking direct thrombin inhibitors or direct factor Xa inhibitors unless laboratory tests such as aPTT, INR, platelet count, ecarin clotting time, thrombin time, or appropriate direct factor Xa activity assays are normal or the patient has not received a dose of these agents for >48 hours (assuming normal renal metabolizing function).
Refer to your local drug formulary for contraindications to the use of tenecteplase.
Candidates for intravenous thrombolysis with alteplase
The AHA/ASA guidelines state that the eligibility recommendations for treating with alteplase are:[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Within 3 hours of stroke symptom onset or patient last known well or at baseline state:
Within 3.0 to 4.5 hours of stroke symptom onset or patient last known well:
Patients aged ≤80 years
Those without a history of both diabetes mellitus and stroke
Those with a baseline National Institutes of Health Stroke Scale (NIHSS) score ≤25
Those not taking any oral anticoagulants
Those without imaging evidence of ischemic injury involving more than one third of the middle cerebral artery territory
Patients whose blood pressure can be lowered safely to <185/110 mmHg with antihypertensive agents
Patients with initial glucose levels >50 mg/dL
Patients with early ischemic changes on noncontrast CT of mild to moderate extent (other than frank hypodensity)
Patients who have had antiplatelet monotherapy or combination therapy before stroke, provided the benefit of alteplase outweighs the possible increased risk of symptomatic intracerebral hemorrhage
Patients with end-stage renal disease on hemodialysis and normal aPTT.
Additional recommendations for treatment with alteplase can be found in the AHA/ASA guidelines.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Normalizing blood glucose
Glucose levels must be >50 mg/dL before initiating intravenous alteplase treatment. For more information, see section on "Supportive care" below.
Lowering blood pressure
Blood pressure must be <185/110 mmHg before initiating intravenous alteplase treatment.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
For more information, see section on "Supportive care" below.
Patients with major deficits and older patients
When treating a patient with major deficits, the likelihood of favorable outcome is reduced and there is increased risk of hemorrhage following thrombolysis.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
The AHA/ASA guidelines state that for otherwise medically eligible patients ≥18 years of age, alteplase administration within 3 hours is equally recommended for patients ≤80 and >80 years of age.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[158]Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021 Mar;6(1):I-LXII.
https://www.doi.org/10.1177/2396987321989865
http://www.ncbi.nlm.nih.gov/pubmed/33817340?tool=bestpractice.com
Although use of alteplase has previously been restricted to people aged under 80 years, evidence shows that patients aged over 80 years derive as much benefit (reduced death or dependency, improved functional outcomes) from alteplase as do those aged under 80 years, especially if treated within 3 hours of stroke.[145]Wardlaw JM, Murray V, Berge E, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014 Jul 29;(7):CD000213.
https://www.doi.org/10.1002/14651858.CD000213.pub3
http://www.ncbi.nlm.nih.gov/pubmed/25072528?tool=bestpractice.com
[159]Bluhmki E, Danays T, Biegert G, et al. Alteplase for acute ischemic stroke in patients aged >80 years: pooled analyses of individual patient data. Stroke. 2020 Aug;51(8):2322-31.
https://www.doi.org/10.1161/STROKEAHA.119.028396
http://www.ncbi.nlm.nih.gov/pubmed/32611284?tool=bestpractice.com
[160]Hacke W, Lyden P, Emberson J, et al. Effects of alteplase for acute stroke according to criteria defining the European Union and United States marketing authorizations: individual-patient-data meta-analysis of randomized trials. Int J Stroke. 2018 Feb;13(2):175-89.
https://www.doi.org/10.1177/1747493017744464
http://www.ncbi.nlm.nih.gov/pubmed/29171359?tool=bestpractice.com
Aspirin and dual antiplatelet therapy
Guidelines recommend that ischemic stroke patients receive aspirin, whether or not they are eligible for alteplase.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
However, if alteplase is given, aspirin should not be started for 24 hours, and only then after a head CT shows the absence of intracranial hemorrhage.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[161]Zinkstok SM, Roos YB; ARTIS investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012 Aug 25;380(9843):731-7.
http://www.ncbi.nlm.nih.gov/pubmed/22748820?tool=bestpractice.com
Early (i.e., within 24 hours) administration of aspirin in acute ischemic stroke patients receiving alteplase did not show any significant improvements in outcomes at 3 months.[161]Zinkstok SM, Roos YB; ARTIS investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012 Aug 25;380(9843):731-7.
http://www.ncbi.nlm.nih.gov/pubmed/22748820?tool=bestpractice.com
Patients with minor stroke
For patients with noncardioembolic ischemic stroke or transient ischemic attack (TIA), guidelines from the AHA/ASA recommend aspirin, clopidogrel, or the combination of aspirin plus extended-release dipyridamole for secondary prevention of ischemic stroke.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
In patients with recent minor (NIHSS score ≤3) noncardioembolic ischemic stroke or high-risk TIA (ABCD2 score ≥4), the AHA/ASA recommend that dual antiplatelet therapy should be initiated early (ideally within 12-24 hours of symptom onset and at least within 7 days of onset) and continued for 21 to 90 days, followed by single antiplatelet therapy, to reduce the risk of recurrent ischemic stroke.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[162]Wang Y, Johnston SC, Bath PM, et al. Acute dual antiplatelet therapy for minor ischaemic stroke or transient ischaemic attack. BMJ. 2019 Feb 28;364:l895.
https://www.bmj.com/content/364/bmj.l895.long
http://www.ncbi.nlm.nih.gov/pubmed/30819687?tool=bestpractice.com
[163]Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007 Nov;6(11):961-9.
http://www.ncbi.nlm.nih.gov/pubmed/17931979?tool=bestpractice.com
The dual antiplatelet therapy regimen of ticagrelor plus aspirin is approved by the Food and Drug Administration (FDA) in the US to reduce the risk for stroke in patients with acute ischemic stroke with a NIHSS score of ≤5 or high-risk TIA. In Europe, an application to the European Medicines Agency (EMA) to change the marketing authorization of ticagrelor to include the prevention of stroke in adults who have had mild to moderate ischemic stroke or high-risk TIA was withdrawn in December 2021. Based on trial data and the company’s response to their questions, the EMA expressed concern that the benefits of short-term treatment with ticagrelor plus aspirin in preventing stroke in these patients did not clearly outweigh the risks of fatal and nonfatal bleeding.
Evidence supporting dual antiplatelet therapy
One meta-analysis found that dual antiplatelet therapy with clopidogrel plus aspirin within 24 hours after minor ischemic stroke (NIHSS score of ≤3; patients not candidates for intravenous thrombolysis) or high-risk TIA reduced the absolute risk of subsequent stroke by 2% compared with aspirin alone.[164]Hao Q, Tampi M, O'Donnell M, et al. Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis. BMJ. 2018 Dec 18;363:k5108.
https://www.bmj.com/content/363/bmj.k5108.long
http://www.ncbi.nlm.nih.gov/pubmed/30563866?tool=bestpractice.com
All-cause mortality did not differ between treatment groups; clopidogrel plus aspirin was associated with a small absolute increased risk of moderate or severe extracranial bleeding (0.2%).[164]Hao Q, Tampi M, O'Donnell M, et al. Clopidogrel plus aspirin versus aspirin alone for acute minor ischaemic stroke or high risk transient ischaemic attack: systematic review and meta-analysis. BMJ. 2018 Dec 18;363:k5108.
https://www.bmj.com/content/363/bmj.k5108.long
http://www.ncbi.nlm.nih.gov/pubmed/30563866?tool=bestpractice.com
In a second meta-analysis, the risk of recurrent ischemic stroke in patients with acute ischemic stroke or TIA was significantly reduced with short-term (≤1 month; RR 0.53, 95% CI 0.37 to 0.78) and intermediate-term (≤3 months; RR 0.72, 95% CI 0.58 to 0.90) aspirin plus clopidogrel compared with aspirin alone, but long-term combination treatment (>3 months) did not reduce risk of recurrent ischemic stroke (RR 0.81, 95% CI 0.63 to 1.04).[165]Rahman H, Khan SU, Nasir F, et al. Optimal duration of aspirin plus clopidogrel after ischemic stroke or transient ischemic attack. Stroke. 2019 Apr;50(4):947-53.
https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.118.023978
http://www.ncbi.nlm.nih.gov/pubmed/30852971?tool=bestpractice.com
Intermediate-term (RR 2.58, 95% CI 1.19 to 5.60) and long-term (RR 1.87, 95% CI 1.36 to 2.56) combined treatment significantly increased the risk of major bleeding, but short-term treatment did not (RR 1.82, 95% CI 0.91 to 3.62).[165]Rahman H, Khan SU, Nasir F, et al. Optimal duration of aspirin plus clopidogrel after ischemic stroke or transient ischemic attack. Stroke. 2019 Apr;50(4):947-53.
https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.118.023978
http://www.ncbi.nlm.nih.gov/pubmed/30852971?tool=bestpractice.com
The THALES trial of 11,016 patients (none of whom received thrombolysis or thrombectomy or required anticoagulation) demonstrated that compared with aspirin alone, dual treatment with ticagrelor plus aspirin reduced the risk of disabling stroke or death within 30 days (4.0% vs. 4.7%).[166]Johnston SC, Amarenco P, Denison H, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020 Jul 16;383(3):207-17.
https://www.doi.org/10.1056/NEJMoa1916870
http://www.ncbi.nlm.nih.gov/pubmed/32668111?tool=bestpractice.com
Severe bleeding was more frequent with ticagrelor plus aspirin than with aspirin alone (0.5% vs. 0.1%), including intracranial hemorrhage (0.4% vs. 0.1%). For patients with recent stroke with an NIHSS score of <5, ticagrelor plus aspirin for 30 days was more effective in preventing recurrent ischemic stroke than aspirin alone.[166]Johnston SC, Amarenco P, Denison H, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020 Jul 16;383(3):207-17.
https://www.doi.org/10.1056/NEJMoa1916870
http://www.ncbi.nlm.nih.gov/pubmed/32668111?tool=bestpractice.com
For patients with an acute ischemic stroke and an NIHSS score of <5, the use of ticagrelor plus aspirin for 30 days reduced recurrent ischemic events in a randomized, placebo-controlled, double-blind trial. However, severe bleeding was more frequent with ticagrelor plus aspirin than with aspirin alone.[166]Johnston SC, Amarenco P, Denison H, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020 Jul 16;383(3):207-17.
https://www.doi.org/10.1056/NEJMoa1916870
http://www.ncbi.nlm.nih.gov/pubmed/32668111?tool=bestpractice.com
In Chinese patients with minor stroke and high risk-TIA (NIHSS score <3) who are carriers of CYP2C19 loss-of-function allele, the use of ticagrelor plus aspirin modestly reduced the risk of stroke at 90 days compared with clopidogrel plus aspirin. The combination treatment was for 21 days followed by either ticagrelor or clopidogrel alone for up to 90 days.[167]Wang Y, Meng X, Wang A, et al. Ticagrelor versus clopidogrel in CYP2C19 loss-of-function carriers with stroke or TIA. N Engl J Med. 2021 Dec 30;385(27):2520-30.
https://www.doi.org/10.1056/NEJMoa2111749
http://www.ncbi.nlm.nih.gov/pubmed/34708996?tool=bestpractice.com
Endovascular interventions
As with alteplase, initiation of endovascular interventions should be carried out as early as possible.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Intravenous thrombolysis with alteplase within 4.5 hours of symptom onset plus mechanical thrombectomy within 6 hours of symptom onset is the standard of care to treat strokes caused by large vessel occlusion (LVO) in patients meeting eligibility criteria.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[168]Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke: from the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). J Vasc Interv Radiol. 2018 Apr;29(4):441-53.
https://www.jvir.org/article/S1051-0443(17)31073-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29478797?tool=bestpractice.com
[169]Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke: endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019 Mar;4(1):6-12.
https://journals.sagepub.com/doi/pdf/10.1177/2396987319832140
http://www.ncbi.nlm.nih.gov/pubmed/31165090?tool=bestpractice.com
[170]Campbell BC, Donnan GA, Mitchell PJ, et al. Endovascular thrombectomy for stroke: current best practice and future goals. Stroke Vasc Neurol. 2016 Feb 16;1(1):16-22.
https://svn.bmj.com/content/1/1/16
http://www.ncbi.nlm.nih.gov/pubmed/28959994?tool=bestpractice.com
Clinical trials and registry data have proven the efficacy of this approach.[171]Rodrigues FB, Neves JB, Caldeira D, et al. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ. 2016 Apr 18;353:i1754.
https://www.bmj.com/content/353/bmj.i1754.long
http://www.ncbi.nlm.nih.gov/pubmed/27091337?tool=bestpractice.com
[172]Mueller-Kronast NH, Zaidat OO, Froehler MT, et al; STRATIS Investigators. Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS Registry. Stroke. 2017 Oct;48(10):2760-8.
https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.117.016456
http://www.ncbi.nlm.nih.gov/pubmed/28830971?tool=bestpractice.com
[173]Vidale S, Agostoni E. Endovascular treatment of ischemic stroke: an updated meta-analysis of efficacy and safety. Vasc Endovascular Surg. 2017 May;51(4):215-9.
http://www.ncbi.nlm.nih.gov/pubmed/28424039?tool=bestpractice.com
[174]Roaldsen MB, Jusufovic M, Berge E, et al. Endovascular thrombectomy and intra-arterial interventions for acute ischaemic stroke. Cochrane Database Syst Rev. 2021 Jun 14;6:CD007574.
https://www.doi.org/10.1002/14651858.CD007574.pub3
http://www.ncbi.nlm.nih.gov/pubmed/34125952?tool=bestpractice.com
The role of thrombectomy alone without intravenous thrombolysis (e.g., where there are contraindications for thrombolysis) has not yet been ascertained. Intra-arterial thrombectomy may be considered without intravenous thrombolysis for:
Patients who present between 4.5 and 6.0 hours after stroke onset[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Patients who present 6-24 hours after stroke onset (last known normal) who meet specific eligibility criteria.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Endovascular interventions include mechanical clot-removing devices, such as stent retrievers, and intra-arterial thrombolysis. The AHA/ASA guidelines recommend the use of stent retrievers over intra-arterial thrombolysis and other mechanical thrombectomy devices (e.g., concentric retrievers) as first-line endovascular therapy for acute ischemic stroke; however, devices other than stent retrievers may be reasonable in some circumstances.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
The use of a proximal balloon guide catheter or large bore distal catheter, rather than a cervical guide catheter alone, in conjunction with stent retrievers may also be useful in certain carefully selected patients.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Furthermore, it may be reasonable to use an adjunctive intervention (e.g., intra-arterial thrombolysis) to achieve acceptable reperfusion, if used within 6 hours of symptom onset.
One systematic review found that, even in patients with mild strokes due to LVO who were not eligible for intravenous thrombolysis with alteplase, mechanical thrombectomy resulted in better 90-day functional outcomes, and suggested that this treatment can play an important role for patients not eligible for intravenous alteplase.[175]Griessenauer CJ, Medin C, Maingard J, et al. Endovascular mechanical thrombectomy in large-vessel occlusion ischemic stroke presenting with low National Institutes of Health Stroke Scale: systematic review and meta-analysis. World Neurosurg. 2018 Feb;110:263-9.
http://www.ncbi.nlm.nih.gov/pubmed/29174232?tool=bestpractice.com
Analysis from three randomized controlled clinical trials (1,092 patients) detected no differences in functional outcomes of intravenous thrombolysis-eligible patients with an acute LVO receiving direct endovascular treatment compared with endovascular treatment preceded by intravenous thrombolysis.[176]Katsanos AH, Turc G, Psychogios M, et al. Utility of intravenous alteplase prior to endovascular stroke treatment: a systematic review and meta-analysis of RCTs. Neurology. 2021 Aug 24;97(8):e777-e784.
http://www.ncbi.nlm.nih.gov/pubmed/34144996?tool=bestpractice.com
The authors noted that because uncertainty for most endpoints remains large and the available data are not able to exclude the possibility of overall benefit or harm, further randomized controlled trials are needed.[176]Katsanos AH, Turc G, Psychogios M, et al. Utility of intravenous alteplase prior to endovascular stroke treatment: a systematic review and meta-analysis of RCTs. Neurology. 2021 Aug 24;97(8):e777-e784.
http://www.ncbi.nlm.nih.gov/pubmed/34144996?tool=bestpractice.com
A subsequent randomized trial (539 patients) found that endovascular treatment alone was neither superior nor noninferior to intravenous alteplase followed by direct endovascular treatment with regard to disability outcome at 90 days after stroke with no difference in the rate of hemorrhage.[177]LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021 Nov 11;385(20):1833-44.
https://www.doi.org/10.1056/NEJMoa2107727
http://www.ncbi.nlm.nih.gov/pubmed/34758251?tool=bestpractice.com
The risk of complications with sequelae for patients from mechanical thrombectomy has been estimated to be around 15%; such complications need to be minimized and effectively managed to maximize the benefits of thrombectomy.[178]Balami JS, White PM, McMeekin PJ, et al. Complications of endovascular treatment for acute ischemic stroke: prevention and management. Int J Stroke. 2018 Jun;13(4):348-61.
http://www.ncbi.nlm.nih.gov/pubmed/29171362?tool=bestpractice.com
General anesthesia during endovascular thrombectomy is associated with worse patient outcomes compared with no general anesthesia (with or without sedation).[179]Campbell BC, van Zwam WH, Goyal M, et al; HERMES collaborators. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018 Jan;17(1):47-53.
http://www.ncbi.nlm.nih.gov/pubmed/29263006?tool=bestpractice.com
Candidates for endovascular interventions
The AHA/ASA guidelines state that patients who are eligible for alteplase should be treated with alteplase even if they are potential candidates for endovascular therapy with a stent retriever.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Observing patients for a clinical response to intravenous alteplase prior to use of endovascular therapy should not be performed.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
The AHA/ASA guidelines suggest that patients who meet all of the following criteria should be treated with a stent retriever:[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Have a prestroke Modified Rankin Disability Scale score 0-1
Have causative occlusion of the internal carotid artery or proximal middle cerebral artery (M1)
Aged ≥18 years
Have an NIHSS score ≥6
Have an Alberta Stroke Program Early CT score (ASPECTS) ≥6
Can begin endovascular therapy (groin puncture) within 6 hours of symptom onset.
Although there is a lack of evidence for stent retrievers in ischemic stroke patients outside these criteria, they may be considered for use in patients with anterior circulation arterial occlusion who cannot be treated with intravenous thrombolysis, or patients with occlusion of other vessels, such as the M2 or M3 portion of the middle cerebral artery, anterior cerebral arteries, vertebral arteries, basilar artery, or posterior cerebral arteries. They may also be considered for patients who are aged <18 years, or have a Modified Rankin Disability Scale score >1, or an ASPECTS <6, if initiated within 6 hours of symptom onset, but the potential benefits are unclear as there is a lack of evidence in these patients.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Regarding thrombectomy, AHA/ASA guidelines state:[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Thrombectomy is recommended within 6-16 hours of "last known well" in selected patients with acute ischemic stroke who have LVO in the anterior circulation and who meet other DAWN or DEFUSE 3 eligibility criteria (see table)
Thrombectomy is reasonable within 16-24 hours of last known well in selected patients with acute ischemic stroke who have LVO in the anterior circulation and who meet other DAWN eligibility criteria (see table).
The DAWN trial used clinical imaging mismatch (combination of NIHSS score and imaging findings on CT perfusion or diffusion-weighted MRI) as eligibility criteria to select patients with LVO in the anterior circulation for mechanical thrombectomy 6-24 hours from last known well.[180]Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018 Jan 4;378(1):11-21.
https://www.nejm.org/doi/10.1056/NEJMoa1706442
http://www.ncbi.nlm.nih.gov/pubmed/29129157?tool=bestpractice.com
The DEFUSE 3 trial used perfusion-core mismatch and maximum core size as imaging criteria to select patients with LVO in the anterior circulation for mechanical thrombectomy 6-16 hours from last known well.[181]Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018 Feb 22;378(8):708-18.
https://www.nejm.org/doi/10.1056/NEJMoa1713973
http://www.ncbi.nlm.nih.gov/pubmed/29364767?tool=bestpractice.com
For patients who otherwise meet criteria for mechanical thrombectomy, noninvasive vessel imaging of the intracranial arteries is recommended during the initial imaging evaluation.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
An appropriate penumbra on CT perfusion or magnetic resonance perfusion imaging is essential before thrombectomy.
Initial treatment with intra-arterial thrombolysis may be considered for carefully selected patients with major ischemic strokes of <6 hours' duration with causative occlusion of the anterior circulation, including the anterior cerebral artery, middle cerebral artery, or distal internal carotid artery, or those with contraindications or an incomplete response to intravenous thrombolysis.[182]Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. JAMA. 1999 Dec 1;282(21):2003-11.
https://jamanetwork.com/journals/jama/fullarticle/192156
http://www.ncbi.nlm.nih.gov/pubmed/10591382?tool=bestpractice.com
However, the evidence for effectiveness is weak, and there are no intra-arterial thrombolytic interventions approved for use in stroke.
[Figure caption and citation for the preceding image starts]: Data from DAWN and DEFUSE-3 trials (cerebral blood flow [CBF]; time-to-maximum [Tmax]; internal carotid artery [ICA]; middle cerebral artery [MCA])Created by BMJ Knowledge Centre using data from Dawn-Nogueira et al. N Engl J Med. 2018 378(1):11-21 and Defuse-Albers et al. N Engl J Med. 2018 22;378(8):708-18 [Citation ends].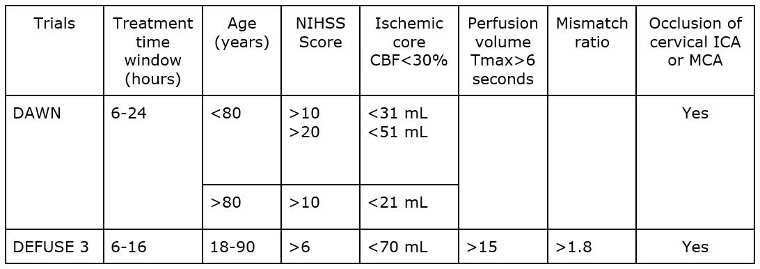
Anticoagulation
Urgent anticoagulation in unselected ischemic stroke patients, with the goal of improving acute stroke outcomes, is generally not recommended.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Meta-analyses failed to show a reduction in stroke disability in acute ischemic stroke patients treated with anticoagulants, but do show an increase in the risk of hemorrhagic transformation of stroke, particularly in patients with larger stroke volumes.[183]Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015 Mar 12;(3):CD000024.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000024.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/25764172?tool=bestpractice.com
[  ]
Is there randomized controlled trial evidence to support the use of anticoagulants after acute ischemic stroke?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3899/fullShow me the answer[Evidence A]764ac23b-a6d7-4124-af2d-1aa10b24f52dccaAIs there randomized controlled trial evidence to support the use of anticoagulants after acute ischemic stroke?
]
Is there randomized controlled trial evidence to support the use of anticoagulants after acute ischemic stroke?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3899/fullShow me the answer[Evidence A]764ac23b-a6d7-4124-af2d-1aa10b24f52dccaAIs there randomized controlled trial evidence to support the use of anticoagulants after acute ischemic stroke?
Although one trial did not find a significant benefit of low-molecular-weight heparin (LMWH) over aspirin in patients with large artery occlusive disease, subgroup analyses and an unblinded randomized controlled trial suggest that LMWH may prevent early neurologic deterioration in subgroups of patients such as older patients and patients with symptomatic posterior circulation arterial disease.[184]Yi X, Lin J, Wang C, et al. Low-molecular-weight heparin is more effective than aspirin in preventing early neurologic deterioration and improving six-month outcome. J Stroke Cerebrovasc Dis. 2014 Jul;23(6):1537-44.
http://www.ncbi.nlm.nih.gov/pubmed/24656240?tool=bestpractice.com
[185]Wang QS, Chen C, Chen XY, et al. Low-molecular-weight heparin versus aspirin for acute ischemic stroke with large artery occlusive disease: subgroup analyses from the Fraxiparin in Stroke Study for the treatment of ischemic stroke (FISS-tris) study. Stroke. 2012 Feb;43(2):346-9.
https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.111.628347
http://www.ncbi.nlm.nih.gov/pubmed/22076004?tool=bestpractice.com
Patients with atrial fibrillation
The optimal time for initiating anticoagulation in patients with atrial fibrillation after acute ischemic stroke or TIA is unclear. The AHA/ASA guidelines recommend starting oral anticoagulation 4 to 14 days after stroke symptom onset.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
In patients with nonvalvular atrial fibrillation and stroke or TIA, oral anticoagulation (e.g., apixaban, edoxaban, rivaroxaban, dabigatran, or warfarin) is recommended to reduce the risk of recurrent stroke, regardless of whether the atrial fibrillation pattern is paroxysmal, persistent, or permanent.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Direct-acting oral anticoagulants (DOACs) such as apixaban, edoxaban, rivaroxaban, or dabigatran are recommended over warfarin in patients with stroke or TIA and atrial fibrillation who do not have moderate to severe mitral stenosis or a mechanical heart valve.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Large randomized trials have shown DOACs to clinically reduce the risk of thrombotic stroke with less bleeding risk compared with vitamin K antagonists (e.g., warfarin).[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
The international normalized ratio (INR) range for patients on warfarin is 2.0 to 3.0.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
[186]Lip GY, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018 Nov;154(5):1121-201.
https://journal.chestnet.org/article/S0012-3692(18)32244-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30144419?tool=bestpractice.com
A validated scoring system should be used to assess the bleeding risk of the patient; if high, the patient should be followed up more closely.[187]Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012 Aug 14;126(7):860-5.
https://www.ahajournals.org/doi/full/10.1161/circulationaha.111.060061
http://www.ncbi.nlm.nih.gov/pubmed/22891166?tool=bestpractice.com
[188]Feldman W. Treatment of occult bacteremia. Pediatrics. 1984 Dec;74(6):1131-3.
http://www.ncbi.nlm.nih.gov/pubmed/6504638?tool=bestpractice.com
See New-onset atrial fibrillation.
Stroke or TIA with stenosis of a major intracranial artery
In patients with a stroke or TIA caused by 50% to 99% stenosis of a major intracranial artery, aspirin is recommended in preference to warfarin to reduce the risk of recurrent ischemic stroke and vascular death.[24]Turan TN, Zaidat OO, Gronseth GS, et al. Stroke prevention in symptomatic large artery intracranial atherosclerosis practice advisory: report of the AAN guideline subcommittee. Neurology. 2022 Mar 22;98(12):486-98.
https://www.doi.org/10.1212/WNL.0000000000200030
http://www.ncbi.nlm.nih.gov/pubmed/35314513?tool=bestpractice.com
[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
In patients with stroke or TIA within 30 days attributable to severe stenosis (70% to 99%) of a major intracranial artery, the addition of clopidogrel to aspirin for up to 90 days is recommended to further reduce recurrent stroke risk in patients who have low risk of hemorrhagic transformation.[24]Turan TN, Zaidat OO, Gronseth GS, et al. Stroke prevention in symptomatic large artery intracranial atherosclerosis practice advisory: report of the AAN guideline subcommittee. Neurology. 2022 Mar 22;98(12):486-98.
https://www.doi.org/10.1212/WNL.0000000000200030
http://www.ncbi.nlm.nih.gov/pubmed/35314513?tool=bestpractice.com
[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
In patients with minor stroke or high-risk TIA within 24 hours and concomitant ipsilateral >30% stenosis of a major intracranial artery, the addition of ticagrelor to aspirin for up to 30 days might be considered to further reduce recurrent stroke risk.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
The dual antiplatelet therapy regimen of ticagrelor plus aspirin is approved in the US by the FDA to reduce the risk for stroke in patients with acute ischemic stroke with a NIHSS score of ≤5 or high-risk TIA. In Europe, an application to the EMA to change the marketing authorization of ticagrelor to include the prevention of stroke in adults who have had mild to moderate ischemic stroke or high-risk TIA was withdrawn in December 2021. Based on trial data and the company’s response to their questions, the EMA expressed concern that the benefits of short-term treatment with ticagrelor plus aspirin in preventing stroke in these patients did not clearly outweigh the risks of fatal and nonfatal bleeding.
Carotid endarterectomy (CEA) and carotid artery stenting (CAS)
In patients with symptomatic carotid stenosis (i.e., TIA or nondisabling stroke) within the past 6 months and ipsilateral severe (70% to 99%) carotid artery stenosis, CEA is recommended to reduce the risk of future stroke. This is appropriate only if perioperative morbidity and mortality risk is estimated to be <6%.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
In patients younger than 68 years and with symptomatic carotid stenosis (i.e., TIA or nondisabling stroke), CAS is preferred over CEA if the degree of stenosis is between 50% and 69% (as determined by digital subtraction angiography). This is appropriate only if perioperative risk of morbidity and mortality is <6%. CEA or CAS is beneficial for patients with 70% to 99% stenosis without near-occlusion. No evidence of benefit has been found in patients with a stenosis of <50% or near-occlusion.[45]Barnett HJ, Taylor DW, Haynes RB, et al; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991 Aug 15;325(7):445-53.
https://www.nejm.org/doi/10.1056/NEJM199108153250701
http://www.ncbi.nlm.nih.gov/pubmed/1852179?tool=bestpractice.com
[46]Barnett HJ, Taylor DW, Eliasziw M, et al; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998 Nov 12;339(20):1415-25.
https://www.nejm.org/doi/10.1056/NEJM199811123392002
http://www.ncbi.nlm.nih.gov/pubmed/9811916?tool=bestpractice.com
[189]Rerkasem A, Orrapin S, Howard DP, et al. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 2020 Sep 12;9:CD001081.
https://www.doi.org/10.1002/14651858.CD001081.pub4
http://www.ncbi.nlm.nih.gov/pubmed/32918282?tool=bestpractice.com
In patients older than 68 years and with TIA or ischemic stroke and ipsilateral moderate (50% to 69%) carotid stenosis (as documented by catheter-based imaging or noninvasive imaging), CEA is recommended to reduce the risk of future stroke. This is appropriate only if perioperative morbidity and mortality risk is <6%. Patient-specific factors such as age, sex, and comorbidities will also affect the suitability of CEA.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
CEA and CAS showed similar benefits in stroke prevention in one randomized controlled trial. Stenting tended to be more effective in patients younger than 68 years, whereas endarterectomy tended to be more effective those in older than 68 years. Stenting was associated with slightly more strokes, and endarterectomy was associated with slightly more myocardial infarctions and 12th cranial nerve damage.[190]Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010 Jul 1;363(1):11-23.
https://www.nejm.org/doi/10.1056/NEJMoa0912321
http://www.ncbi.nlm.nih.gov/pubmed/20505173?tool=bestpractice.com
The durability of carotid artery stenting was proven at 10-year follow-up; survival rate was slightly lower in the stenting group than in the endarterectomy group (probably due to periprocedural differences in risk).[191]Brott TG, Howard G, Roubin GS, et al; CREST Investigators. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016 Mar 17;374(11):1021-31.
https://www.nejm.org/doi/10.1056/NEJMoa1505215
http://www.ncbi.nlm.nih.gov/pubmed/26890472?tool=bestpractice.com
See Carotid artery stenosis.
Patent foramen ovale (PFO)
PFO closure (with antiplatelet therapy), antiplatelet therapy alone, or anticoagulants alone are options for the secondary prevention of stroke in patients with cryptogenic ischemic stroke secondary to PFO.[192]Messé SR, Gronseth GS, Kent DM, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the guideline subcommittee of the American Academy of Neurology. Neurology. 2020 May 19;94(20):876-85.
https://www.doi.org/10.1212/WNL.0000000000009443
http://www.ncbi.nlm.nih.gov/pubmed/32350058?tool=bestpractice.com
Antiplatelet options include aspirin or clopidogrel.[193]Kuijpers T, Spencer FA, Siemieniuk RA, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation therapy alone for management of cryptogenic stroke? A clinical practice guideline. BMJ. 2018 Jul 25;362:k2515.
https://www.bmj.com/content/362/bmj.k2515.long
http://www.ncbi.nlm.nih.gov/pubmed/30045912?tool=bestpractice.com
In patients with a high risk of paradoxical embolism (RoPE) score, closure of the PFO reduces stroke recurrence compared with medical treatment alone.[194]Ahmed N, Steiner T, Caso V, et al; ESO-KSU session participants. Recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 13-15 November 2016. Eur Stroke J. 2017 Jun;2(2):95-102.
http://journals.sagepub.com/doi/full/10.1177/2396987317699144
[195]Ahmad Y, Howard JP, Arnold A, et al. Patent foramen ovale closure vs. medical therapy for cryptogenic stroke: a meta-analysis of randomized controlled trials. Eur Heart J. 2018 May 7;39(18):1638-49.
https://academic.oup.com/eurheartj/article/39/18/1638/4944510
http://www.ncbi.nlm.nih.gov/pubmed/29590333?tool=bestpractice.com
[196]Lattanzi S, Brigo F, Cagnetti C, et al. Patent foramen ovale and cryptogenic stroke or transient ischemic attack: to close or not to close? A systematic review and meta-analysis. Cerebrovasc Dis. 2018;45(5-6):193-203.
https://www.karger.com/Article/FullText/488401
http://www.ncbi.nlm.nih.gov/pubmed/29649819?tool=bestpractice.com
[197]Ntaios G, Papavasileiou V, Sagris D, et al. Closure of patent foramen ovale versus medical therapy in patients with cryptogenic stroke or transient ischemic attack: updated systematic review and meta-analysis. Stroke. 2018 Feb;49(2):412-8.
https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.117.020030
http://www.ncbi.nlm.nih.gov/pubmed/29335335?tool=bestpractice.com
[198]Vidale S, Russo F, Campana C, et al. Patent foramen ovale closure versus medical therapy in cryptogenic strokes and transient ischemic attacks: a meta-analysis of randomized trials. Angiology. 2019 Apr;70(4):325-31.
http://www.ncbi.nlm.nih.gov/pubmed/30270651?tool=bestpractice.com
In patients younger than 60 years with a PFO and embolic-appearing infarct and no other mechanism of stroke identified, clinicians may recommend closure. This decision should always include a discussion of potential benefits (absolute recurrent stroke risk reduction of 3.4% at 5 years) and risks (periprocedural complication rate of 3.9% and increased absolute rate of non-periprocedural atrial fibrillation of 0.33% per year).[192]Messé SR, Gronseth GS, Kent DM, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the guideline subcommittee of the American Academy of Neurology. Neurology. 2020 May 19;94(20):876-85.
https://www.doi.org/10.1212/WNL.0000000000009443
http://www.ncbi.nlm.nih.gov/pubmed/32350058?tool=bestpractice.com
PFO closure may be considered in other populations, such as for a patient who is aged 60-65 years with a very limited degree of traditional vascular risk factors (i.e., hypertension, diabetes, hyperlipidemia, or smoking) and no other mechanism of stroke detected following a thorough evaluation, including prolonged monitoring for atrial fibrillation.[192]Messé SR, Gronseth GS, Kent DM, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the guideline subcommittee of the American Academy of Neurology. Neurology. 2020 May 19;94(20):876-85.
https://www.doi.org/10.1212/WNL.0000000000009443
http://www.ncbi.nlm.nih.gov/pubmed/32350058?tool=bestpractice.com
All patients with previous stroke should be treated with an antithrombotic medication indefinitely if there is no bleeding contraindication, regardless of whether a PFO is present or if it is closed.
For patients aged under 60 years, PFO closure plus antiplatelet therapy is likely to be of benefit for secondary stroke prevention compared with anticoagulant therapy.[193]Kuijpers T, Spencer FA, Siemieniuk RA, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation therapy alone for management of cryptogenic stroke? A clinical practice guideline. BMJ. 2018 Jul 25;362:k2515.
https://www.bmj.com/content/362/bmj.k2515.long
http://www.ncbi.nlm.nih.gov/pubmed/30045912?tool=bestpractice.com
PFO closure plus antiplatelet therapy is preferred to antiplatelet therapy alone if anticoagulation is contraindicated or declined.[193]Kuijpers T, Spencer FA, Siemieniuk RA, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation therapy alone for management of cryptogenic stroke? A clinical practice guideline. BMJ. 2018 Jul 25;362:k2515.
https://www.bmj.com/content/362/bmj.k2515.long
http://www.ncbi.nlm.nih.gov/pubmed/30045912?tool=bestpractice.com
[199]Mir H, Siemieniuk RA, Ge L, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation in patients with patent foramen ovale and cryptogenic stroke: a systematic review and network meta-analysis incorporating complementary external evidence. BMJ Open. 2018 Jul 25;8(7):e023761.
https://bmjopen.bmj.com/content/8/7/e023761.long
http://www.ncbi.nlm.nih.gov/pubmed/30049703?tool=bestpractice.com
Atrial fibrillation is more frequent in patients who have their PFO closed, but is mostly transient.[197]Ntaios G, Papavasileiou V, Sagris D, et al. Closure of patent foramen ovale versus medical therapy in patients with cryptogenic stroke or transient ischemic attack: updated systematic review and meta-analysis. Stroke. 2018 Feb;49(2):412-8.
https://www.ahajournals.org/doi/full/10.1161/STROKEAHA.117.020030
http://www.ncbi.nlm.nih.gov/pubmed/29335335?tool=bestpractice.com
[199]Mir H, Siemieniuk RA, Ge L, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation in patients with patent foramen ovale and cryptogenic stroke: a systematic review and network meta-analysis incorporating complementary external evidence. BMJ Open. 2018 Jul 25;8(7):e023761.
https://bmjopen.bmj.com/content/8/7/e023761.long
http://www.ncbi.nlm.nih.gov/pubmed/30049703?tool=bestpractice.com
See Patent foramen ovale.
Patients with cerebral venous sinus thrombosis (CVST)
CVST can cause intracerebral venous hemorrhage, ischemic stroke, brain edema, midline shift, and elevation of intracranial pressure. Treatment with anticoagulation should begin as soon as the diagnosis of CVST is confirmed.[8]Saposnik G, Bushnell C, Coutinho JM, et al. Diagnosis and management of cerebral venous thrombosis: a scientific statement from the American Heart Association. Stroke. 2024 Mar;55(3):e77-90.
https://www.ahajournals.org/doi/10.1161/STR.0000000000000456
http://www.ncbi.nlm.nih.gov/pubmed/38284265?tool=bestpractice.com
[83]Ulivi L, Squitieri M, Cohen H, et al. Cerebral venous thrombosis: a practical guide. Pract Neurol. 2020 Oct;20(5):356-67.
https://www.doi.org/10.1136/practneurol-2019-002415
http://www.ncbi.nlm.nih.gov/pubmed/32958591?tool=bestpractice.com
Specialist guidance should be sought on whether to choose low molecular weight heparin (LMWH) or unfractionated heparin. The American Heart Association and European Stroke Organisation preferentially suggest an LMWH over unfractionated heparin due to the more practical administration, more predictable anticoagulation effect, lower risk of thrombocytopenia, efficacy of LMWH and lower rates of hemorrhagic complications.[8]Saposnik G, Bushnell C, Coutinho JM, et al. Diagnosis and management of cerebral venous thrombosis: a scientific statement from the American Heart Association. Stroke. 2024 Mar;55(3):e77-90.
https://www.ahajournals.org/doi/10.1161/STR.0000000000000456
http://www.ncbi.nlm.nih.gov/pubmed/38284265?tool=bestpractice.com
[144]Ferro JM, Bousser MG, Canhão P, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur J Neurol. 2017 Oct;24(10):1203-13.
https://onlinelibrary.wiley.com/doi/10.1111/ene.13381
http://www.ncbi.nlm.nih.gov/pubmed/28833980?tool=bestpractice.com
The presence of venous hemorrhage does not constitute a contraindication for anticoagulation.[8]Saposnik G, Bushnell C, Coutinho JM, et al. Diagnosis and management of cerebral venous thrombosis: a scientific statement from the American Heart Association. Stroke. 2024 Mar;55(3):e77-90.
https://www.ahajournals.org/doi/10.1161/STR.0000000000000456
http://www.ncbi.nlm.nih.gov/pubmed/38284265?tool=bestpractice.com
[200]Zuurbier SM, Arnold M, Middeldorp S, et al. Risk of cerebral venous thrombosis in obese women. JAMA Neurol. 2016 May 1;73(5):579-84.
https://www.doi.org/10.1001/jamaneurol.2016.0001
http://www.ncbi.nlm.nih.gov/pubmed/26974867?tool=bestpractice.com
[201]Fan Y, Yu J, Chen H, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of cerebral venous sinus thrombosis. Stroke Vasc Neurol. 2020 Jun;5(2):152-8.
https://www.doi.org/10.1136/svn-2020-000358
http://www.ncbi.nlm.nih.gov/pubmed/32409571?tool=bestpractice.com
For subsequent prevention of CVST, the treatment duration depends on the number of episodes of CVST and if there is a known underlying cause identified. Treatment duration should be discussed with a hematologist. Oral anticoagulants used for CVST include vitamin K antagonists such as warfarin (INR range 2.0 to 3.0), and DOACs. DOACs appear to be a safe and effective alternative option to VKAs according to open-label retrospective and prospective randomized studies.[8]Saposnik G, Bushnell C, Coutinho JM, et al. Diagnosis and management of cerebral venous thrombosis: a scientific statement from the American Heart Association. Stroke. 2024 Mar;55(3):e77-90.
https://www.ahajournals.org/doi/10.1161/STR.0000000000000456
http://www.ncbi.nlm.nih.gov/pubmed/38284265?tool=bestpractice.com
[202]Ferro JM, Coutinho JM, Dentali F, et al. Safety and efficacy of dabigatran etexilate vs dose-adjusted Wwarfarin in patients with cerebral venous thrombosis: a randomized clinical trial. JAMA Neurol. 2019 Dec 1;76(12):1457-65.
https://www.doi.org/10.1001/jamaneurol.2019.2764
http://www.ncbi.nlm.nih.gov/pubmed/31479105?tool=bestpractice.com
[203]Field TS, Dizonno V, Almekhlafi MA, et al. Study of rivaroxaban for cerebral venous thrombosis: a randomized controlled feasibility trial comparing anticoagulation with rivaroxaban to standard-of-care in symptomatic cerebral venous thrombosis. Stroke. 2023 Nov;54(11):2724-36.
https://www.doi.org/10.1161/STROKEAHA.123.044113
http://www.ncbi.nlm.nih.gov/pubmed/37675613?tool=bestpractice.com
[204]Yaghi S, Shu L, Bakradze E, et al. Direct oral anticoagulants versus warfarin in the treatment of cerebral venous thrombosis (ACTION-CVT): a multicenter international study. Stroke. 2022 Mar;53(3):728-38.
https://www.doi.org/10.1161/STROKEAHA.121.037541
http://www.ncbi.nlm.nih.gov/pubmed/35143325?tool=bestpractice.com
[205]Yaghi S, Saldanha IJ, Misquith C, et al. Direct oral anticoagulants versus vitamin K antagonists in cerebral venous thrombosis: a systematic review and meta-analysis. Stroke. 2022 Oct;53(10):3014-24.
https://www.doi.org/10.1161/STROKEAHA.122.039579
http://www.ncbi.nlm.nih.gov/pubmed/35938419?tool=bestpractice.com
In select cases of CVST, endovascular therapies (direct thrombectomy or intra-clot thrombolysis with r-tPA) may be considered by a multidisciplinary team.[83]Ulivi L, Squitieri M, Cohen H, et al. Cerebral venous thrombosis: a practical guide. Pract Neurol. 2020 Oct;20(5):356-67.
https://www.doi.org/10.1136/practneurol-2019-002415
http://www.ncbi.nlm.nih.gov/pubmed/32958591?tool=bestpractice.com
Given the lack of controlled studies (and poorer outcomes in meta-analyses), endovascular therapies are reserved for patients with evidence of thrombus propagation, for individuals with neurologic deterioration despite medical therapy, or for those with contraindications to anticoagulation.[8]Saposnik G, Bushnell C, Coutinho JM, et al. Diagnosis and management of cerebral venous thrombosis: a scientific statement from the American Heart Association. Stroke. 2024 Mar;55(3):e77-90.
https://www.ahajournals.org/doi/10.1161/STR.0000000000000456
http://www.ncbi.nlm.nih.gov/pubmed/38284265?tool=bestpractice.com
Statins
Statin therapy with intensive lipid-lowering effects is recommended for patients with ischemic stroke or TIA, to lower the risk of stroke and cardiovascular events.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
[206]Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Jun 18;139(25):e1082-143.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000625
http://www.ncbi.nlm.nih.gov/pubmed/30586774?tool=bestpractice.com
Statin treatment should not be started immediately. There is consensus that it is safe to start statins after 48 hours.[133]National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. April 2022 [internet publication].
https://www.nice.org.uk/guidance/ng128
Statin treatment should be continued in people who are already receiving statins.[133]National Institute for Health and Care Excellence. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management. April 2022 [internet publication].
https://www.nice.org.uk/guidance/ng128
There is evidence that the rate of recurrent cardiovascular events or stroke is lower in patients whose low-density lipoprotein (LDL) is controlled to <70 mg/dL compared with those with LDL between 90 and 110 mg/dL.[24]Turan TN, Zaidat OO, Gronseth GS, et al. Stroke prevention in symptomatic large artery intracranial atherosclerosis practice advisory: report of the AAN guideline subcommittee. Neurology. 2022 Mar 22;98(12):486-98.
https://www.doi.org/10.1212/WNL.0000000000200030
http://www.ncbi.nlm.nih.gov/pubmed/35314513?tool=bestpractice.com
[207]Amarenco P, Kim JS, Labreuche J, et al. A Comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020 Jan 2;382(1):9.
https://www.doi.org/10.1056/NEJMoa1910355
http://www.ncbi.nlm.nih.gov/pubmed/31738483?tool=bestpractice.com
Monitoring of liver enzymes is recommended for patients taking statins. Caution should be exercised when prescribing high-intensity statins to patients with a history of intracerebral hemorrhage.
In patients with ischemic stroke with no known coronary heart disease, no major cardiac sources of embolism, and LDL cholesterol (LDL-C) >100 mg/dL, atorvastatin is indicated to reduce risk of stroke recurrence.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
In patients with stroke or TIA and hyperlipidemia, adherence to changes in lifestyle and the effects of LDL-C–lowering medication should be assessed by measurement of fasting lipids and appropriate safety indicators 4-12 weeks after statin initiation or dose adjustment. This assessment should be repeated every 3-12 months thereafter, based on need to assess adherence or safety.[102]Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021 Jul;52(7):e364-467.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000375
http://www.ncbi.nlm.nih.gov/pubmed/34024117?tool=bestpractice.com
Supportive care
At the same time as acute evaluation for thrombolysis and/or thrombectomy, the following steps should be taken:
Support blood oxygenation. Supplemental oxygen should be provided only when blood oxygen saturation is <94%. Liberal use of oxygen is associated with increased mortality in acutely sick patients.[208]Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018 Apr 28;391(10131):1693-705.
http://www.ncbi.nlm.nih.gov/pubmed/29726345?tool=bestpractice.com
[209]Siemieniuk RAC, Chu DK, Kim LH, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
https://www.bmj.com/content/363/bmj.k4169.long
http://www.ncbi.nlm.nih.gov/pubmed/30355567?tool=bestpractice.com
Patients with decreased level of consciousness or refractory hypoxemia may require intubation with mechanical ventilation.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[210]Rønning OM, Guldvog B. Should stroke victims routinely receive supplemental oxygen? A quasi-randomized controlled trial. Stroke. 1999 Oct;30(10):2033-7.
https://www.ahajournals.org/doi/full/10.1161/01.str.30.10.2033
http://www.ncbi.nlm.nih.gov/pubmed/10512903?tool=bestpractice.com
Support systemic blood pressure. Management of arterial blood pressure in acute ischemic stroke is controversial because of conflicting evidence and a lack of large controlled clinical trials.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Many patients with ischemic stroke have elevated blood pressure at presentation. Lowering blood pressure could reduce cerebral perfusion pressure and promote stroke extension.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[214]Ahmed N, Näsman P, Wahlgren NG. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke. 2000 Jun;31(6):1250-5.
https://www.ahajournals.org/doi/full/10.1161/01.str.31.6.1250
http://www.ncbi.nlm.nih.gov/pubmed/10835440?tool=bestpractice.com
However, AHA/ASA guidelines recommend early treatment of hypertension when required by comorbid conditions. Particular comorbidities include concomitant acute coronary event, acute heart failure, aortic dissection, post-fibrinolysis sICH [symptomatic intracerebral hemorrhage], or preeclampsia/eclampsia. Management of these patients should be individualized, but in general, initial blood pressure reduction by 15% is a reasonable goal.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Blood pressure of <185/110 mmHg is required before initiating intravenous alteplase. Intensive lowering of systolic blood pressure to 130-140 mmHg within 1 hour before alteplase treatment was found to be safe, but it did not appear to improve clinical outcomes compared with a target of <180 mmHg.[215]Anderson CS, Huang Y, Lindley RI, et al; ENCHANTED Investigators and Coordinators. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (ENCHANTED): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet. 2019 Mar 2;393(10174):877-88.
http://www.ncbi.nlm.nih.gov/pubmed/30739745?tool=bestpractice.com
[216]Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021 Jun;6(2):XLVIII-LXXXIX.
https://www.doi.org/10.1177/23969873211012133
http://www.ncbi.nlm.nih.gov/pubmed/34780578?tool=bestpractice.com
Normalize blood glucose levels (required before initiating intravenous alteplase).
Hypoglycemia can cause brain injury and should be avoided. One randomized trial found that in acute ischemic stroke patients with hyperglycemia, aggressive control of glucose levels with an intravenous insulin drip did not result in a significant difference in favorable functional outcome at 90 days compared with standard glucose control, but it was associated with severe hypoglycemia in more patients (2.6%).[217]Johnston KC, Bruno A, Pauls Q, et al; Neurological Emergencies Treatment Trials Network and the SHINE Trial Investigators. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA. 2019 Jul 23;322(4):326-35.
https://jamanetwork.com/journals/jama/fullarticle/2738553
http://www.ncbi.nlm.nih.gov/pubmed/31334795?tool=bestpractice.com
Hypoglycemia can be well controlled with frequent subcutaneous insulin injections based on a sliding scale.[218]Fuentes B, Ntaios G, Putaala J, et al. European Stroke Organisation (ESO) guidelines on glycaemia management in acute stroke. Eur Stroke J. 2018 Mar;3(1):5-21.
https://journals.sagepub.com/doi/full/10.1177/2396987317742065
http://www.ncbi.nlm.nih.gov/pubmed/31008333?tool=bestpractice.com
Hyperglycemia has been associated with poor outcome and risk of hemorrhagic transformation of ischemic stroke.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[139]Baird TA, Parsons MW, Phanh T, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003 Sep;34(9):2208-14.
https://www.ahajournals.org/doi/full/10.1161/01.str.0000085087.41330.ff
http://www.ncbi.nlm.nih.gov/pubmed/12893952?tool=bestpractice.com
[140]Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001 Nov 13;57(9):1603-10.
http://www.ncbi.nlm.nih.gov/pubmed/11706099?tool=bestpractice.com
[141]Demchuk AM, Morgenstern LB, Krieger DW, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999 Jan;30(1):34-9.
https://www.ahajournals.org/doi/full/10.1161/01.str.30.1.34
http://www.ncbi.nlm.nih.gov/pubmed/9880385?tool=bestpractice.com
Treatment of significantly elevated blood glucose is recommended since evidence indicates that persistent in-hospital hyperglycemia during the first 24 hours after acute ischemic stroke is associated with worse outcomes than normoglycemia.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Reduce fever. Fever may be associated with poor stroke outcome.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[219]Saxena M, Young P, Pilcher D, et al. Early temperature and mortality in critically ill patients with acute neurological diseases: trauma and stroke differ from infection. Intensive Care Med. 2015 May;41(5):823-32.
https://www.doi.org/10.1007/s00134-015-3676-6
http://www.ncbi.nlm.nih.gov/pubmed/25643903?tool=bestpractice.com
Treatment of fever is therefore reasonable, although not yet shown to be effective by controlled trials.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[220]Krieger DW, Yenari MA. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke. 2004 Jun;35(6):1482-9.
https://www.ahajournals.org/doi/full/10.1161/01.str.0000126118.44249.5c
http://www.ncbi.nlm.nih.gov/pubmed/15073396?tool=bestpractice.com
[221]Den Hertog HM, van der Worp HB, Tseng MC, et al. Cooling therapy for acute stroke. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD001247.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001247.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/19160194?tool=bestpractice.com
[222]Ntaios G, Dziedzic T, Michel P, et al. European Stroke Organisation (ESO) guidelines for the management of temperature in patients with acute ischemic stroke. Int J Stroke. 2015 Aug;10(6):941-9.
https://journals.sagepub.com/doi/full/10.1111/ijs.12579
http://www.ncbi.nlm.nih.gov/pubmed/26148223?tool=bestpractice.com
These steps, while not shown to be effective in clinical trials, may retard stroke evolution or prevent stroke extension by optimizing energy substrate delivery and tissue energy metabolism.
Following emergency department evaluation and treatment, patients with ischemic stroke should be transferred to a dedicated stroke unit. These units improve stroke functional outcome and survival.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[223]Langhorne P, Ramachandra S, Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev. 2020 Apr 23;(4):CD000197.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000197.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/32324916?tool=bestpractice.com
[224]Adeoye O, Nyström KV, Yavagal DR, et al. Recommendations for the establishment of stroke systems of care: a 2019 update. Stroke. 2019 Jul;50(7):e187-210.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000173
http://www.ncbi.nlm.nih.gov/pubmed/31104615?tool=bestpractice.com
[  ]
How does organized inpatient care compare with care on a general medical ward for people with stroke?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3108/fullShow me the answer Stroke units should have multidisciplinary teams which include physicians, nursing staff, and rehabilitation specialists with expertise in stroke. Improved supportive care, avoidance of complications such as infection, and earlier initiation of rehabilitation therapy are among the mechanisms by which stroke units are hypothesized to produce better outcomes.
]
How does organized inpatient care compare with care on a general medical ward for people with stroke?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3108/fullShow me the answer Stroke units should have multidisciplinary teams which include physicians, nursing staff, and rehabilitation specialists with expertise in stroke. Improved supportive care, avoidance of complications such as infection, and earlier initiation of rehabilitation therapy are among the mechanisms by which stroke units are hypothesized to produce better outcomes.
Nutritional support, rehabilitation therapy (physical, occupational, and/or speech therapy as indicated), prevention of aspiration (swallowing assessment), and prevention of deep vein thrombosis (DVT)/venous thromboembolism (VTE) are all required in the subacute phase of hospital care.
[  ]
In people with aphasia following stroke, how does the use of speech and language therapy affect outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1384/fullShow me the answer
[
]
In people with aphasia following stroke, how does the use of speech and language therapy affect outcomes?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1384/fullShow me the answer
[  ]
Does electromechanical and robot‐assisted arm training improve generic activities of daily living, arm function, and arm strength in patients who have had a stroke?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2317/fullShow me the answer
]
Does electromechanical and robot‐assisted arm training improve generic activities of daily living, arm function, and arm strength in patients who have had a stroke?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2317/fullShow me the answer
Swallowing impairment is common in stroke and is associated with an increased risk of aspiration pneumonia and death.[225]Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005 Dec;36(12):2756-63.
https://www.ahajournals.org/doi/full/10.1161/01.str.0000190056.76543.eb
http://www.ncbi.nlm.nih.gov/pubmed/16269630?tool=bestpractice.com
[226]Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999 Apr;30(4):744-8.
https://www.ahajournals.org/doi/full/10.1161/01.str.30.4.744
http://www.ncbi.nlm.nih.gov/pubmed/10187872?tool=bestpractice.com
Guidelines support the use of a bedside swallow test before eating or drinking but do not provide specifics on test administration and interpretation.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
A reasonable approach is to withhold oral intake if there is coughing or a wet voice after swallowing a small cup of water. Patients who cannot take nutrition orally should receive isotonic fluids (to decrease risk of brain edema) and have enteral feeding by nasogastric, nasoduodenal, or percutaneous gastrostomy tube.
[  ]
How does percutaneous endoscopic gastrostomy compare with nasogastric tube feeding in people with swallowing disturbances?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1134/fullShow me the answer
]
How does percutaneous endoscopic gastrostomy compare with nasogastric tube feeding in people with swallowing disturbances?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1134/fullShow me the answer
VTE prophylaxis
VTE is the cause of about 10% of stroke deaths.[227]Wijdicks EF, Scott JP. Pulmonary embolism associated with acute stroke. Mayo Clin Proc. 1997 Apr;72(4):297-300.
http://www.ncbi.nlm.nih.gov/pubmed/9121173?tool=bestpractice.com
Intermittent pneumatic compression of the legs is recommended to reduce the risk of DVT/VTE in nonambulatory stroke patients.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[228]Dennis M, Caso V, Kappelle LJ, et al; European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur Stroke J. 2016 Mar;1(1):6-19.
https://journals.sagepub.com/doi/full/10.1177/2396987316628384
http://www.ncbi.nlm.nih.gov/pubmed/31008263?tool=bestpractice.com
[229]Dennis M, Sandercock P, Reid J, et al; CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013 Aug 10;382(9891):516-24.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61050-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/23727163?tool=bestpractice.com
Elastic compression stockings are not recommended.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[228]Dennis M, Caso V, Kappelle LJ, et al; European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur Stroke J. 2016 Mar;1(1):6-19.
https://journals.sagepub.com/doi/full/10.1177/2396987316628384
http://www.ncbi.nlm.nih.gov/pubmed/31008263?tool=bestpractice.com
The benefits of prophylactic subcutaneous heparin in patients with acute ischemic stroke are not well established; it decreases the rates of DVT and pulmonary embolism, but is also associated with a significant increase in the rate of hemorrhage, with no significant effect on mortality or functional status at final follow-up.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Guidelines note that there may be a subgroup of patients for whom the benefits of reducing the risk of VTE with heparin outweighs the increased risk of intracranial and extracranial bleeding.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[228]Dennis M, Caso V, Kappelle LJ, et al; European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur Stroke J. 2016 Mar;1(1):6-19.
https://journals.sagepub.com/doi/full/10.1177/2396987316628384
http://www.ncbi.nlm.nih.gov/pubmed/31008263?tool=bestpractice.com
There is no prediction tool to identify these patients, but patients considered to be at particularly high risk of VTE include those with complete paralysis of the leg, previous VTE, dehydration or comorbidities (such as malignancy or sepsis), or current or recent smokers.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[228]Dennis M, Caso V, Kappelle LJ, et al; European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur Stroke J. 2016 Mar;1(1):6-19.
https://journals.sagepub.com/doi/full/10.1177/2396987316628384
http://www.ncbi.nlm.nih.gov/pubmed/31008263?tool=bestpractice.com
Early mobilization is recommended for stroke patients, but very early, intense mobilization (e.g., multiple out-of-bed sessions) within 24 hours of stroke onset should not be performed.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
[230]AVERT Trial Collaboration group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015 Jul 4;386(9988):46-55.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)60690-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/25892679?tool=bestpractice.com
Early mobilization may decrease risk of VTE by reducing venous stasis, but this has not been demonstrated in controlled trials.[228]Dennis M, Caso V, Kappelle LJ, et al; European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur Stroke J. 2016 Mar;1(1):6-19.
https://journals.sagepub.com/doi/full/10.1177/2396987316628384
http://www.ncbi.nlm.nih.gov/pubmed/31008263?tool=bestpractice.com
See Venous thromboembolism prophylaxis.
Rehabilitation
Early rehabilitation after stroke is recommended.[231]Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016 Jun;47(6):e98-169.
https://www.ahajournals.org/doi/full/10.1161/str.0000000000000098
http://www.ncbi.nlm.nih.gov/pubmed/27145936?tool=bestpractice.com
However, high intensity, very early mobilization within 24 hours of stroke onset should not be performed because it can reduce the odds of a favorable outcome at 3 months.[119]Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-418.
https://www.ahajournals.org/doi/full/10.1161/STR.0000000000000211
http://www.ncbi.nlm.nih.gov/pubmed/31662037?tool=bestpractice.com
Because of neurologic injury, many stroke patients have limited ambulation and mobility, which reduces quality of life.
The objective of rehabilitation is to enable the person to return to an acceptable social and/or working life.
Speech and language therapy is crucial in order to increase the degree of functional communication. About one third of patients who have a stroke develop aphasia.[232]Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17(1):35-43.
https://www.doi.org/10.1159/000073896
http://www.ncbi.nlm.nih.gov/pubmed/14530636?tool=bestpractice.com
Differences in functional outcome when comparing specific therapy regimens (i.e., intensity, dosage, and duration) are being investigated. It is known that people who have highly intensive treatments have a higher incidence of dropping out of therapy early.[233]Brady MC, Kelly H, Godwin J, et al. Speech and language therapy for aphasia following stroke. Cochrane Database Syst Rev. 2016 Jun 1;(6):CD000425.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000425.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/27245310?tool=bestpractice.com
Mental practice describes a training method that uses cognitive rehearsal of activities to improve performance of those activities; an individual repeatedly mentally rehearses an action or task in their imagination (e.g., picking up a cup or reaching out with their arm) without physically performing the action or task. Randomized controlled trials support the use of mental practice, in addition to conventional physical rehabilitation treatment, in improving upper extremity impairment after stroke.[234]Barclay RE, Stevenson TJ, Poluha W, et al. Mental practice for treating upper extremity deficits in individuals with hemiparesis after stroke. Cochrane Database Syst Rev. 2020 May 25;5:CD005950.
https://www.doi.org/10.1002/14651858.CD005950.pub5
http://www.ncbi.nlm.nih.gov/pubmed/32449959?tool=bestpractice.com
Virtual reality and interactive video gaming have emerged as novel treatment approaches in stroke rehabilitation. One meta-analysis found that virtual reality may improve upper limb function and activities of daily living when used as an adjunct to usual care (to increase overall therapy time); however, virtual reality and interactive video gaming was not more beneficial than conventional therapy. There was insufficient evidence to reach conclusions about the effect of virtual reality and interactive video gaming on gait speed, balance, participation, or quality of life.[235]Laver KE, Lange B, George S, et al. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2017 Nov 20;(11):CD008349.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008349.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/29156493?tool=bestpractice.com
Gait Exercise Assist Robot (GEAR) may produce clinically significant improvements in balance and lower extremity function in patients with infratentorial stroke.[236]Tomida K, Sonoda S, Hirano S, et al. Randomized controlled trial of gait training using Gait Exercise Assist Robot (GEAR) in stroke patients with hemiplegia. J Stroke Cerebrovasc Dis. 2019 Sep;28(9):2421-8.
http://www.ncbi.nlm.nih.gov/pubmed/31307899?tool=bestpractice.com

 ]
[Evidence A]
]
[Evidence A] ]
Stroke units should have multidisciplinary teams which include physicians, nursing staff, and rehabilitation specialists with expertise in stroke. Improved supportive care, avoidance of complications such as infection, and earlier initiation of rehabilitation therapy are among the mechanisms by which stroke units are hypothesized to produce better outcomes.
]
Stroke units should have multidisciplinary teams which include physicians, nursing staff, and rehabilitation specialists with expertise in stroke. Improved supportive care, avoidance of complications such as infection, and earlier initiation of rehabilitation therapy are among the mechanisms by which stroke units are hypothesized to produce better outcomes. ]
[
]
[  ]
]
 ]
]