Approach
ATN can present in all medical settings but is more frequently acquired in hospital.[5] The condition is often asymptomatic and only diagnosed by laboratory tests. A history of hypotension, fluid depletion, or exposure to nephrotoxic agents is usually present. Suggestive history and physical findings supported by blood tests, urine studies, and a renal ultrasound confirms the diagnosis.
History
ATN is more likely to develop in patients with poor renal perfusion (e.g., renal transplantation, renal surgery, or renal artery thrombosis), underlying renal disease (e.g., myeloma), diabetes mellitus, or pre-existing hypovolaemia. Excessive fluid loss (due to haemorrhage, burns, gastrointestinal losses, or sweating) may present with symptoms of hypovolaemia, including thirst. They may also have poor oral intake, malaise, and anorexia. There may be a history of sepsis or pancreatitis. ATN may present after haemorrhage, drug overdose, surgery, cardiac arrest, or other conditions with hypotension and prolonged renal ischaemia. Orthopnoea and paroxysmal nocturnal dyspnoea may occur if advanced cardiac failure is present leading to reduced renal perfusion.
Patients may have history of exposure to radiocontrast media or nephrotoxic drugs (e.g., aminoglycosides, amphotericin-B, non-steroidal anti-inflammatory drugs, or chemotherapeutic agents [e.g., cisplatin]). They may have history of muscle trauma, gout, or tumour lysis syndrome.
Physical examination
Physical examination findings may be unremarkable because the condition is often asymptomatic and only diagnosed by laboratory tests. The condition is suspected when serum creatinine increases above 26.5 micromol/L/day (0.3 mg/dL/day) within 48 hours after a hypotensive event or exposure to a nephrotoxin. Patients with fluid loss, sepsis, or pancreatitis may have hypotension, tachycardia, oliguria, or anuria together with other signs of circulatory collapse (e.g., oedema). Prolonged ATN also can result in bleeding due to dysfunctional platelets.
Initial laboratory investigations
Initial work-up should include basic metabolic profile (including urea and creatinine), urine chemistries (for fractional excretion of sodium and urea, osmolality), urinalysis, and full blood count.
Serum chemistries
Elevated urea and creatinine levels are indicators of ATN; however, they are not sensitive markers of glomerular filtration rate (GFR) and can be affected by nutritional state, muscle mass, use of steroids, or presence of gastrointestinal bleeding. An increase in creatinine levels can be influenced by other non-renal events, such as rhabdomyolysis.[5] Creatinine is also a late marker of injury; when there is a rise in serum creatinine, it occurs 48-72 hours after the event. Both urea and creatinine levels become abnormal when the GFR decreases by more than 50%. In ATN, the urea:creatinine ratio is usually 10:1. In a pre-renal situation, urea reabsorption at the proximal tubule is increased; therefore, the ratio rises from 10:1 to 20:1 or higher.[2]
High serum potassium and metabolic acidosis suggests ATN. Hyperkalaemia is most pronounced in individuals with excessive endogenous potassium production, such as in rhabdomyolysis, haemolysis, and tumour lysis syndrome.
Measurement of creatine kinase and free myoglobin are used to identify possible rhabdomyolysis. The risk of acute kidney injury (AKI) associated with rhabdomyolysis is usually low when creatine kinase levels are less than 15,000 to 20,000 U/L. However, it may appear with low levels (as low as 5000 U/L) if there are other coexisting conditions such as sepsis, dehydration, or acidosis.[26]
Urinalysis
Examination of urine sediment can be useful to differentiate ATN from other conditions, such as pre-renal conditions. A purely pre-renal cause of AKI results in a sediment characterised by hyaline casts, while in ATN, the sediment may show pigmented, muddy brown, granular casts, implying the presence of tubular injury and necrosis. The higher the number of casts, the more severe the AKI, the more likely the need for dialysis therapy, and the higher the mortality rate.[27]
Haem-positive urine in the absence of erythrocytes in the sediment suggests ATN from haemolysis or rhabdomyolysis.
Uric acid crystals in urine does not confirm a diagnosis of uric acid nephropathy as it may be present in samples of healthy patients. However, uric acid crystalluria can be observed in patients with rhabdomyolysis or lymphoproliferative disorders complicated by a tumour lysis syndrome.[27] Sulfadiazine or methotrexate crystals can also be observed precipitating in the urine when these drugs damage the kidney.
Urine indexes
Tubule dysfunction leads to increased urinary sodium concentration, as the injured tubules are unable to increase reabsorption of water, sodium, and urea.[5]
Impairment in urinary concentrating capacity is characterised by a decrease in urine osmolality.[5] This can help differentiate between pre-renal azotaemia (in which the re-absorptive capacity and concentrating ability of the kidney are preserved or enhanced) and ATN (in which these functions are impaired).
Fractional excretion of sodium (FENa) over 2% supports a diagnosis of ATN.[5] Tubule dysfunction leads to increased fractional excretion of sodium. However, an FENa over 2% is also expected with the use of diuretics, chronic kidney disease (CKD), after intravenous fluid therapy, and in glucosuria or bicarbonaturia. In contrast, some cases of ATN, such as in haemolysis, rhabdomyolysis, or contrast-induced AKI can have FENa <1%.[28] The FENa is calculated as follows: (urine sodium × plasma creatinine)/(plasma sodium × urine creatinine) × 100%.
Fractional excretion of urea (FEUrea) can be a more reliable index in cases of diuretic use or CKD. An FEUrea over 35% is expected in ATN.[29] It is calculated as follows: (urine urea × plasma creatinine)/(plasma urea × urine creatinine) × 100%.
Elevated urinary myoglobin levels suggest ATN from rhabdomyolysis.
Blood studies
Anaemia should not be expected in every case of ATN unless the predisposing cause justifies it. Anaemia is expected in multiple myeloma, blood loss, haemolysis, or previous CKD.
Prolonged ATN can also result in bleeding as a result of dysfunctional platelets. There are good data supporting the theory that impaired platelet function is one of the main determinants of uraemic bleeding. This impairment is multifactorial and includes defects intrinsic to the platelet as well as abnormal platelet-endothelial interaction. Uraemic toxins and anaemia also play a role.
Subsequent tests
Arterial blood gases (ABG) are measured to confirm metabolic acidosis.
ECG is performed if hyperkalaemia is suspected or detected by laboratory tests. It may demonstrate arrhythmias if hyperkalaemia is present. Atrioventricular block may be seen if hyperkalaemia is severe.
A renal ultrasound evaluates post-obstructive causes as well as in renal architecture and size. It is also useful for diagnosis of underlying CKD.
The measurement of the inferior vena cava (IVC) diameter can also be used to assess volume status. Measured 3 cm from the right atrium, the normal diameter of IVC is 1.5 to 2.5 cm. Volume depletion is considered with an IVC diameter <1.5 cm, while an IVC diameter >2.5 cm suggests volume overload.[30]
A renal ultrasound is ordered to assist in evaluation of post-obstructive causes as well as in the evaluation of renal architecture and size. It is also useful for diagnosis of underlying chronic kidney disease.
Biopsy should only be performed when the history, clinical features, and findings of laboratory and radiological investigation suggest primary renal disease other than ischaemic or toxic-related ATN.[5] Once a diagnosis of ATN has been made, biopsy can also be used to establish the renal prognosis (degree of tubulo-interstitial fibrosis and/or glomerulosclerosis). Histology of ATN demonstrates denuded basement membranes and presence of cells in the tubule lumen, focal areas of proximal tubule vacuolisation and flattening, tubular dilatation, and brush border debris present in tubular lumen. [Figure caption and citation for the preceding image starts]: Denuded basement membranes and presence of cells in the tubule lumenCourtesy of Puigvert Foundation, Barcelona, Spain [Citation ends].
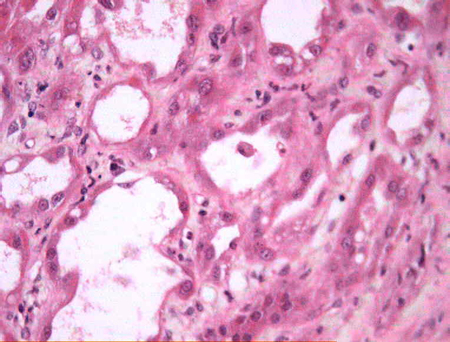 [Figure caption and citation for the preceding image starts]: Focal areas of proximal tubule vacuolisation and flattening. Tubular dilatation. Brush border debris is present in some tubular lumenCourtesy of Puigvert Foundation, Barcelona, Spain [Citation ends].
[Figure caption and citation for the preceding image starts]: Focal areas of proximal tubule vacuolisation and flattening. Tubular dilatation. Brush border debris is present in some tubular lumenCourtesy of Puigvert Foundation, Barcelona, Spain [Citation ends].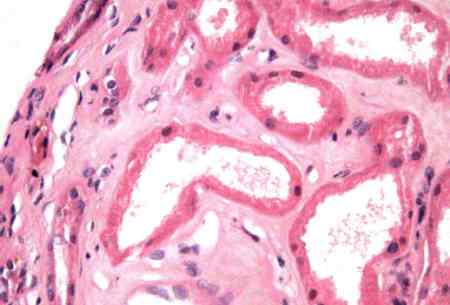
Various novel serum and urinary biomarkers are showing potential as useful indicators for the diagnosis, classification, and prognosis of AKI. They could potentially be able to differentiate between pre-renal azotaemia and acute tubular injury.[31][32] Kidney injury molecule-1 (KIM-1) has been shown to be a good early biomarker of ischaemic ATN.[33][34] Other biomarkers include neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-18 (IL-18).[35][36] Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) are G1 cell cycle arrest biomarkers. They have been found to be effective in predicting the need for renal replacement therapy and mortality in patients at high risk for AKI.[37] Unfortunately, these biomarkers are still undergoing research and have not yet been widely introduced into clinical practice.[38]
Use of this content is subject to our disclaimer