History and exam
Key diagnostic factors
common
presence of risk factors
Including poorly controlled diabetes with or without ketoacidosis, bone marrow or solid organ transplant, graft-versus-host disease, haematological malignancy, corticosteroid therapy, iron overload, iron chelation therapy with desferrioxamine, malnourishment, prematurity in newborns, burns, traumatic inoculation, and inoculation from illicit intravenous drug use.[7]
sinus and facial pain
Common presentation in rhino-orbito-cerebral disease. Usually a manifestation of sinusitis in association with facial pain, facial numbness, and, occasionally, bloody nasal discharge.[3]
eye pain, blurred vision
Common presentation in rhino-orbito-cerebral disease. Result of involvement of the optic nerve or optic artery.
proptosis
Common in rhino-orbito-cerebral disease. Accompanied by marked chemosis and ophthalmoplegia. Associated with fungal infiltration of the orbit.[3]
cranial nerve palsies
Common in rhino-orbito-cerebral disease. Involvement of cranial nerves is an ominous sign and indicates invasion into the central nervous system. Ophthalmoplegia commonly results from the spread of untreated infection from the ethmoid sinuses. Involvement of the contra-lateral eye signifies cavernous sinus thrombosis.
dry cough, with or without dyspnoea
Typically a dry cough (with or without dyspnoea), fever, and chest pain after stem cell transplantation are common in pulmonary mucormycosis.
However, the more common cause, especially in a haematological patient, is aspergillosis. But suspect mucormycosis as the cause of the cough, in the event of no improvement, worsening of symptoms or appearance on imaging studies, and if the patient is receiving appropriate therapy for aspergillosis with a drug not active against agents of mucormycosis.
skin nodules
Common manifestation of cutaneous and disseminated mucormycosis.
Skin involvement in the cutaneous form is more commonly a locally invasive disease extending to the soft tissue, fascia, muscles, and even bone, requiring extensive debridement for good outcomes. This deep extension occurs in about 44% of patients with cutaneous disease.[39] Usually presents in otherwise immunocompetent patients as a result of traumatic inoculation, dressings, or burns.
Nodules are more common when skin is involved as a part of disseminated disease. Mortality can be >80% in very locally invasive disease in an immunocompromised host without extensive surgery.[3]
Other diagnostic factors
common
fever
Common in pulmonary, gastrointestinal, and disseminated disease. Fever is absent in about one-half the cases of rhino-orbito-cerebral mucormycosis.[3]
peri-orbital cellulitis
Common in rhino-orbito-cerebral disease.[3][Figure caption and citation for the preceding image starts]: Peri-orbital mucormycosisFrom the CDC Public Health Image Library (PHIL): Dr Thomas F. Sellers, Emory University [Citation ends].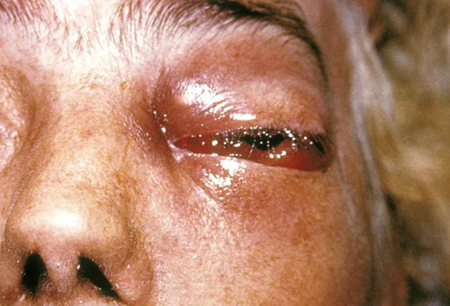
viscid, dark brown-black nasal discharge
Common in rhino-orbito-cerebral disease.[45]
focal sensory/motor neurological deficits and altered mental status
Thrombosis of the internal carotid artery can lead to neurological deficits and altered mental status.
necrotic eschar
Presence on skin, palate, or nasal turbinates is common in the later stages of infection. Due to vessel thrombosis by the invading fungus and subsequent tissue infarction. Absence early in the disease does not rule out mucormycosis.
uncommon
haemoptysis
Can be massive and fatal. Death usually results from dissemination rather than respiratory failure in untreated cases except in haemoptysis. Overall mortality is high, 50% to 70%, and is >95% in disseminated disease.[3]
abdominal pain and distension
Non-specific to gastrointestinal mucormycosis, which is rare except in extremely malnourished people and premature neonates.
gastrointestinal bleeding
Mucormycosis may cause ulceration and bleeding.
Necrotising enterocolitis occurs in premature neonates.
Risk factors
strong
diabetes mellitus ± diabetic ketoacidosis
Increased incidence of mucormycosis, especially rhino-orbito-cerebral disease. Mechanism of action is not entirely clear. Absence of ketoacidosis in a diabetic patient with sinusitis does not rule out mucormycosis. At least 50% of diabetic patients with rhino-orbito-cerebral mucormycosis are not ketoacidotic.
haematological malignancy
A significant risk factor for mucormycosis due to the immune dysregulation caused by both the disease and its treatment.[29] Patients with leukaemia, lymphoma, and myelodysplastic syndromes often have impaired phagocytic function, defects in innate immunity, and disrupted mucosal barriers, all of which facilitate fungal invasion.[29][30] Chemotherapy increases susceptibility by inducing cytopenias (e.g., prolonged neutropenia) and mucosal damage, providing an entry point for Mucorales. Iron overload from frequent transfusions creates a favourable environment for fungal growth. As a result, mucormycosis is a severe and often fatal complication in this patient population, with mortality rates ranging from 40% to 80%.[31]
neutropenia
Adequate and functioning neutrophils are essential in killing Mucorales by oxidative and non-oxidative mechanisms. Hence, patients with neutropenia are at an increased risk of developing mucormycosis. The risk increases with the increase in duration of neutropenia. Recovery of the neutrophil count with granulocyte-colony stimulating factor may help improve outcomes, but the role of these haematopoietic growth factors in modifying the clinical course of mucormycosis is understudied.[32]
iron overload or use of desferrioxamine
Iron overload states and the use of desferrioxamine, but not hydroxypyridinone iron chelators (e.g., deferiprone, deferasirox), are strong risk factors for the development of mucormycosis. Iron promotes growth of Rhizopus. While desferrioxamine is an iron chelator, it also acts as a xenosiderophore increases the availability of iron by forming a complex with iron.[28]
Dialysis patients treated with desferrioxamine are at greater risk than patients with normal renal function. This could be explained by the prolonged half-life of desferrioxamine in patients with renal failure.[28]
use of corticosteroids
Corticosteroids have pleiotropic effects on macrophages and neutrophils that impair their phagocytic function and enhance the susceptibility of corticosteroid-treated patients to mucormycosis. Corticosteroids interfere with the ability of Rhizopus oryzae hyphae to induce production of toxic phagocytic products and activity of oxidative metabolites.[33] However, the mechanism of action remains unknown.
haematopoietic and solid organ transplantation, graft-versus-host disease
Induction chemotherapy and corticosteroids cause quantitative and functional impairment of phagocytes, thereby increasing susceptibility to mucormycosis.
breakdown of skin and soft tissue
Disruption of the skin and/or soft-tissue barrier as in catheter insertion, surgical incisions, and burns allows a portal of entry for Mucorales, resulting in invasive local disease, especially in the immunosuppressed host. Traumatic inoculation of spores after motor vehicle accidents or injuries during natural disasters can also cause the disease in immunocompetent patients.[18][34] The use of illicit drugs also increases the risk of inoculation of spores.
malnutrition
weak
liver cirrhosis
Case reports have shown that mucormycosis is associated with high mortality in patients with liver cirrhosis, despite aggressive treatment of the infection.[36]
Use of this content is subject to our disclaimer