Postmenopausal women
Before starting pharmacotherapy for osteoporosis, secondary causes of bone loss should be excluded; pharmacotherapy for postmenopausal osteoporosis is recommended for patients who are at high risk of fracture, who meet any of these criteria:[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
T-score -2.5 or lower by dual-energy x-ray absorptiometry (DXA) of the femoral neck, total hip, lumbar spine, or 33% radius
History of fragility fracture, including asymptomatic vertebral fracture
T-score between -1.0 and -2.5 and increased risk of fracture, as determined by a formal clinical risk assessment tool
If patients meet these criteria, consideration of the use of osteoporosis pharmacotherapy in postmenopausal women should include the following:[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
Type of treatment
Timing of initiation
Length of treatment
Use of drug holidays to reduce the adverse effects
Bone loss management when therapy is discontinued
Timing of therapy reinitiation
Indications for referral to an endocrinologist or other osteoporosis specialist
[Figure caption and citation for the preceding image starts]: Management of postmenopausal women with non-glucocorticoid-induced osteoporosisCreated by BMJ Knowledge Centre [Citation ends].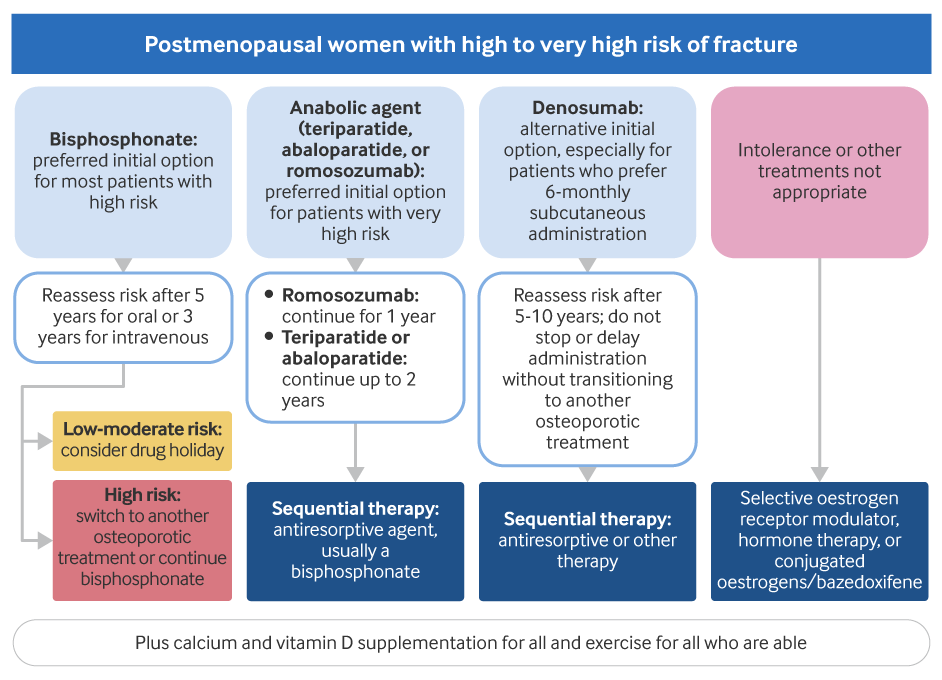
Bisphosphonates
Bisphosphonates (e.g., alendronic acid, ibandronic acid, risedronate, and zoledronic acid) are indicated for the treatment of osteoporosis in postmenopausal women.[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
[109]Rosenberg K. Bisphosphonates likely beneficial for most older women with osteoporosis. Am J Nurs. 2022 Apr 1;122(4):57.
http://www.ncbi.nlm.nih.gov/pubmed/35348523?tool=bestpractice.com
They effectively increase BMD and decrease risk of vertebral and non-vertebral fractures.[110]Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009 Feb;122(2 Suppl):S14-21.
http://www.ncbi.nlm.nih.gov/pubmed/19187808?tool=bestpractice.com
[111]Zhao S, Zhao W, Du D, et al. Effect of bisphosphonate on hip fracture in patients with osteoporosis or osteopenia according to age: a meta-analysis and systematic review. J Investig Med. 2022 Mar;70(3):837-43.
https://journals.sagepub.com/doi/10.1136/jim-2021-001961
http://www.ncbi.nlm.nih.gov/pubmed/34893517?tool=bestpractice.com
This is with the exception of ibandronic acid, which has only been shown to decrease the risk of femoral fracture in high-risk populations with a femoral neck BMD T-score of -3 or less by post-hoc analysis.[112]Chesnut CH 3rd, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004 Aug;19(8):1241-9.
https://onlinelibrary.wiley.com/doi/full/10.1359/JBMR.040325
http://www.ncbi.nlm.nih.gov/pubmed/15231010?tool=bestpractice.com
Bisphosphonates are the first-line treatment for postmenopausal women at high risk of fracture with prior hip or vertebral fractures and/or DXA T-score of ≤-2.5. It is recommended that treatment commences at the time of diagnosis.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
Bisphosphonates may also be used for women with a very high risk of fracture (e.g., very low T-score and a history of severe or multiple vertebral fractures, a recent fracture, or multiple risk factors for fracture), but guidelines recommend initial treatment with an anabolic agent as the preferred option for these patients.[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
[81]Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in post-menopausal women and men >50 years of age. Mar 2024 [internet publication].
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/executive-summary
[113]Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020 Mar 1;105(3):dgaa048.
https://academic.oup.com/jcem/article/105/3/587/5739968
http://www.ncbi.nlm.nih.gov/pubmed/32068863?tool=bestpractice.com
[114]Tai TW, Chen HY, Shih CA, et al. Asia-Pacific consensus on long-term and sequential therapy for osteoporosis. Osteoporos Sarcopenia. 2024 Mar;10(1):3-10.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11056428
http://www.ncbi.nlm.nih.gov/pubmed/38690538?tool=bestpractice.com
Oral bisphosphonates reduce the risk of fracture in women with prior fragility fractures.[115]Saito T, Sterbenz JM, Malay S, et al. Effectiveness of anti-osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta-analysis. Osteoporos Int. 2017 Dec;28(12):3289-300.
http://www.ncbi.nlm.nih.gov/pubmed/28770272?tool=bestpractice.com
[116]Lee SY, Jung SH, Lee SU, et al. Can bisphosphonates prevent recurrent fragility fractures? A systematic review and meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2018 May;19(5):384-90.
http://www.ncbi.nlm.nih.gov/pubmed/29704927?tool=bestpractice.com
A drug holiday for patients with a low to moderate risk of fracture should be considered for patients:[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
who are stable after 5 years of treatment with oral bisphosphonates, or
after 3 years of treatment with zoledronic acid.
For patients at high risk of fracture, longer treatment of up to 10 years with oral bisphosphonates or up to 6 years with zoledronic acid is suggested.[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
Adverse effects of oral bisphosphonates primarily relate to the upper gastrointestinal tract and include difficulty swallowing, oesophagitis, and gastric ulcers. Joint and muscular pain, osteonecrosis of the jaw, and atypical femoral fractures have also been reported.[117]Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014 Jan;29(1):1-23.
https://onlinelibrary.wiley.com/doi/full/10.1002/jbmr.1998
http://www.ncbi.nlm.nih.gov/pubmed/23712442?tool=bestpractice.com
[118]Abrahamsen B. Adverse effects of bisphosphonates. Calcif Tissue Int. 2010 Jun;86(6):421-35.
http://www.ncbi.nlm.nih.gov/pubmed/20407762?tool=bestpractice.com
[119]Lee S, Yin RV, Hirpara H, et al. Increased risk for atypical fractures associated with bisphosphonate use. Fam Pract. 2015 Jun;32(3):276-81.
http://fampra.oxfordjournals.org/content/32/3/276.long
http://www.ncbi.nlm.nih.gov/pubmed/25846215?tool=bestpractice.com
[120]Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint and muscle pain. Arch Intern Med. 2005 Feb 14;165(3):346-7.
http://www.ncbi.nlm.nih.gov/pubmed/15710802?tool=bestpractice.com
[121]Khan AA, Sándor GK, Dore E, et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009 Mar;36(3):478-90.
http://www.ncbi.nlm.nih.gov/pubmed/19286860?tool=bestpractice.com
[122]Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006 May 16;144(10):753-61.
http://www.ncbi.nlm.nih.gov/pubmed/16702591?tool=bestpractice.com
One large systematic review and meta-analysis of more than 650,000 patients demonstrated an increased risk of atypical fractures with bisphosphonates.[119]Lee S, Yin RV, Hirpara H, et al. Increased risk for atypical fractures associated with bisphosphonate use. Fam Pract. 2015 Jun;32(3):276-81.
http://fampra.oxfordjournals.org/content/32/3/276.long
http://www.ncbi.nlm.nih.gov/pubmed/25846215?tool=bestpractice.com
Further research concluded that the benefits of bisphosphonate treatment outweigh the risk of atypical femur fracture, particularly in patients treated for 3-5 years.[123]Black DM, Abrahamsen B, Bouxsein ML, et al. Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr Rev. 2019 Apr 1;40(2):333-68.
https://academic.oup.com/edrv/article/40/2/333/5082430
http://www.ncbi.nlm.nih.gov/pubmed/30169557?tool=bestpractice.com
Consensus is emerging about strategies to prevent atypical femur fracture in patients treated with bisphosphonates, including drug holidays after 5 years' use in some patients.[123]Black DM, Abrahamsen B, Bouxsein ML, et al. Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr Rev. 2019 Apr 1;40(2):333-68.
https://academic.oup.com/edrv/article/40/2/333/5082430
http://www.ncbi.nlm.nih.gov/pubmed/30169557?tool=bestpractice.com
Alendronic acid and risedronate:[124]Barrionuevo P, Kapoor E, Asi N, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. 2019 May 1;104(5):1623-30.
https://academic.oup.com/jcem/article/104/5/1623/5418882
http://www.ncbi.nlm.nih.gov/pubmed/30907957?tool=bestpractice.com
[125]Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a
randomized trial. JAMA. 2006 Dec 27;296(24):2927-38.
http://jama.jamanetwork.com/article.aspx?articleid=204789
http://www.ncbi.nlm.nih.gov/pubmed/17190893?tool=bestpractice.com
[126]Schwartz AV, Bauer DC, Cummings SR, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res. 2010 May;25(5):976-82.
https://onlinelibrary.wiley.com/doi/10.1002/jbmr.11
http://www.ncbi.nlm.nih.gov/pubmed/20200926?tool=bestpractice.com
[127]Roux C, Seeman E, Eastell R, et al. Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin. 2004 Apr;20(4):433-9.
http://www.ncbi.nlm.nih.gov/pubmed/15119979?tool=bestpractice.com
[128]Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008 Mar;19(3):365-72.
http://www.ncbi.nlm.nih.gov/pubmed/17938986?tool=bestpractice.com
[129]Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010 Apr;95(4):1555-65.
http://www.ncbi.nlm.nih.gov/pubmed/20173017?tool=bestpractice.com
Alendronic acid and risedronate were the first bisphosphonates approved for treatment of postmenopausal osteoporosis and remain among the most commonly prescribed osteoporosis treatments. Both have been shown to reduce the risk of vertebral, non-vertebral, and hip fractures.
In one placebo-controlled extension trial, women who continued alendronic acid treatment for 10 years had a significantly lower risk of clinically recognised vertebral fractures compared with those who discontinued alendronic acid after 5 years. The risk of non-vertebral fractures was not significantly different between the two groups overall, but a post-hoc analysis found that continuing alendronic acid for 10 years reduced the risk of non-vertebral fractures in a sub-group of women with a T-score of ≤-2.5 and no vertebral fractures after 5 years of treatment. There was no benefit to continuing alendronic acid beyond 5 years in those with a T-score >-2.0.
Analysis of the Vertebral Efficacy with Risedronate Therapy (VERT) trials suggests that risedronate reduces the risk of vertebral fractures within 6 months of treatment. Clinical trials support the sustained efficacy of risedronate through 5-7 years of treatment and a continued anti-fracture benefit for 1 year after stopping treatment.
Ibandronic acid:[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
[130]Adami S, Felsenberg D, Christiansen C, et al. Efficacy and safety of ibandronate given by
intravenous injection once every 3 months. Bone. 2004 May;34(5):881-9.
http://www.ncbi.nlm.nih.gov/pubmed/15121020?tool=bestpractice.com
[131]Stakkestad JA, Benevolenskaya LI, Stepan JJ, et al; Ibandronate Intravenous Study Group. Intravenous ibandronate injections given every three months: a new treatment
option to prevent bone loss in postmenopausal women. Ann Rheum Dis. 2003 Oct;62(10):969-75.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1754320
http://www.ncbi.nlm.nih.gov/pubmed/12972476?tool=bestpractice.com
May reduce vertebral fracture but its effects in reducing hip fracture and non-vertebral fracture are uncertain, and it is therefore not recommended for these indications in postmenopausal women.
Intravenous ibandronic acid has been shown to be effective in increasing BMD in the treatment and prevention of postmenopausal osteoporosis.
Zoledronic acid:[132]Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012 Feb;27(2):243-54.
https://asbmr.onlinelibrary.wiley.com/doi/full/10.1002/jbmr.1494
http://www.ncbi.nlm.nih.gov/pubmed/22161728?tool=bestpractice.com
[133]Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2015 May;30(5):934-44.
https://onlinelibrary.wiley.com/doi/full/10.1002/jbmr.2442
http://www.ncbi.nlm.nih.gov/pubmed/25545380?tool=bestpractice.com
[134]Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007 May 3;356(18):1809-22.
https://www.nejm.org/doi/10.1056/NEJMoa067312
http://www.ncbi.nlm.nih.gov/pubmed/17476007?tool=bestpractice.com
[135]Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006 May;38(5):617-27.
http://www.ncbi.nlm.nih.gov/pubmed/16046206?tool=bestpractice.com
[136]MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008 Feb 5;148(3):197-213.
http://annals.org/article.aspx?articleid=739219
http://www.ncbi.nlm.nih.gov/pubmed/18087050?tool=bestpractice.com
[137]Sharma A, Einstein AJ, Vallakati A, et al. Risk of atrial fibrillation with use of oral and intravenous bisphosphonates. Am J Cardiol. 2014 Jun 1;113(11):1815-21.
http://www.ncbi.nlm.nih.gov/pubmed/24837258?tool=bestpractice.com
[138]Pazianas M, Compston J, Huang CL. Atrial fibrillation and bisphosphonate therapy. J Bone Miner Res. 2010 Jan;25(1):2-10.
https://onlinelibrary.wiley.com/doi/full/10.1359/jbmr.091201
http://www.ncbi.nlm.nih.gov/pubmed/20091928?tool=bestpractice.com
[139]Reid IR, Horne AM, Mihov B, et al. Duration of fracture prevention after zoledronate treatment in women with osteopenia: observational follow-up of a 6-year randomised controlled trial to 10 years. Lancet Diabetes Endocrinol. 2024 Apr;12(4):247-56.
http://www.ncbi.nlm.nih.gov/pubmed/38452783?tool=bestpractice.com
[140]Liu S, Tan Y, Huang W, et al. Cardiovascular safety of zoledronic acid in the treatment of primary osteoporosis: a meta-analysis and systematic review. Semin Arthritis Rheum. 2024 Feb;64:152304.
http://www.ncbi.nlm.nih.gov/pubmed/37984227?tool=bestpractice.com
One study demonstrated that 6 years of treatment with zoledronic acid maintained BMD, decreased vertebral fracture, and reduced bone turnover markers compared with 3 years of treatment in patients with osteoporosis. In an extension study, those who were treated with zoledronic acid for 6 years were randomised to either continue zoledronic acid for a further 3 years or switched to placebo. The extension study found no significant decrease in the number of fractures between the two groups. In an observational follow-up of older women with osteopenia randomised to receive zoledronic acid or placebo at 18-month intervals for 6 years, reduced fracture rates were maintained for 1.5 to 3.5 years after the last zoledronic acid infusion, but were similar to the placebo group thereafter.
Zoledronic acid exerts a faster effect in the prevention of vertebral fractures and a slower onset effect in the prevention of hip fractures.
Conflicting results have been reported concerning the risk of serious atrial fibrillation in women treated with zoledronic acid. Systematic reviews of RCTs and observational studies showed significantly increased risk of new-onset atrial fibrillation with both intravenous and oral bisphosphonates. However, studies did not show evidence of increased comorbidities such as stroke or death. Overall, the risk-benefit balance outweighs such complications in this population.
Denosumab
Denosumab is a fully human monoclonal antibody against receptor activator of nuclear factor-kappa B ligand (RANKL), a cytokine that is involved in mediating osteoclast activity.
It is approved for the treatment of:
Postmenopausal women with osteoporosis who are at high risk for fracture
Osteoporosis prophylaxis in women at high risk for fracture after receiving adjuvant aromatase inhibitor therapy for breast cancer.
Denosumab may also be used for women with a very high risk of fracture (e.g., very low T-score and a history of severe or multiple vertebral fractures, a recent fracture, or multiple risk factors for fracture), but guidelines recommend initial treatment with an anabolic agent as the preferred option for these patients.[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
[81]Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in post-menopausal women and men >50 years of age. Mar 2024 [internet publication].
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/executive-summary
[113]Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020 Mar 1;105(3):dgaa048.
https://academic.oup.com/jcem/article/105/3/587/5739968
http://www.ncbi.nlm.nih.gov/pubmed/32068863?tool=bestpractice.com
[114]Tai TW, Chen HY, Shih CA, et al. Asia-Pacific consensus on long-term and sequential therapy for osteoporosis. Osteoporos Sarcopenia. 2024 Mar;10(1):3-10.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11056428
http://www.ncbi.nlm.nih.gov/pubmed/38690538?tool=bestpractice.com
Denosumab should be used with caution in patients with pre-existing hypocalcaemia or who are at high risk of hypocalcaemia (vitamin D deficient), and in patients with severe renal disease, including end-stage renal disease.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
The US Food and Drug Administration (FDA) has warned of an increased risk of severe hypocalcaemia in patients with advanced chronic kidney disease who are receiving denosumab.[141]Food and Drug Administration. FDA adds boxed warning for increased risk of severe hypocalcemia in patients with advanced chronic kidney disease taking osteoporosis medicine Prolia (denosumab). Jan 2025 [internet publication].
https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-severe-hypocalcemia-patients-advanced-chronic-kidney-disease
Two safety studies showed a significant increase in the risk of severe hypocalcaemia in patients treated with denosumab compared with those treated with bisphosphonates, with the highest risk reported in patients with advanced kidney disease, particularly those on dialysis.[141]Food and Drug Administration. FDA adds boxed warning for increased risk of severe hypocalcemia in patients with advanced chronic kidney disease taking osteoporosis medicine Prolia (denosumab). Jan 2025 [internet publication].
https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-severe-hypocalcemia-patients-advanced-chronic-kidney-disease
[142]Bird ST, Smith ER, Gelperin K, et al. Severe hypocalcemia with denosumab among older female dialysis-dependent patients. JAMA. 2024 Feb 13;331(6):491-9.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10799290
http://www.ncbi.nlm.nih.gov/pubmed/38241060?tool=bestpractice.com
Severe hypocalcaemia was more common in those with a mineral and bone disorder. Patients with advanced chronic kidney disease taking denosumab are at risk of serious outcomes from severe hypocalcaemia, including hospitalisation and death. Before prescribing denosumab, healthcare professionals should assess kidney function and calcium levels, and consider other treatment options for patients at risk. During treatment, frequent monitoring and prompt management of hypocalcaemia are essential.
The American College of Obstetrics and Gynecology recommends:[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
Denosumab as initial therapy for postmenopausal patients at increased risk of fracture who prefer 6-monthly subcutaneous administration
Patients who discontinue denosumab therapy should be transitioned to treatment with another antiresorptive agent.
The Endocrine Society recommends:[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
Denosumab as an alternative initial treatment in postmenopausal women with osteoporosis who are at high risk for osteoporotic fractures.
The effects of denosumab on bone remodelling, reflected in bone turnover markers, reverse after 6 months if the drug is not taken on schedule. Therefore, a drug holiday or treatment interruption is not recommended with this agent.
In postmenopausal women with osteoporosis taking denosumab, administration should not be delayed or stopped without subsequent antiresorptive therapy (e.g., bisphosphonate, hormone therapy, or SERM) or other therapy administered to prevent a rebound in bone turnover and to decrease the risk of rapid BMD loss and an increased risk of fracture.
Fracture risk should be reassessed after 5-10 years of treatment and those women with a high risk of fracture should continue treatment with denosumab or be treated with other osteoporotic treatment.
The American College of Physicians recommends denosumab as a second-line treatment to reduce the risk of fracture in postmenopausal women with primary osteoporosis who have contraindications to or experience adverse effects with bisphosphonates.[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
The FREEDOM trial demonstrated that denosumab given for 36 months was associated with reduction in the risk of vertebral, non-vertebral, and hip fractures in women with osteoporosis who had a BMD T-score of -2.5 but not less than -4.0 at the lumbar spine or total hip, compared with placebo.[143]Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009 Aug 20;361(8):756-65.
http://www.nejm.org/doi/full/10.1056/NEJMoa0809493#t=article
http://www.ncbi.nlm.nih.gov/pubmed/19671655?tool=bestpractice.com
Extension studies of the FREEDOM trial reported that:[144]Papapoulos S, Lippuner K, Roux C, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int. 2015 Dec;26(12):2773-83.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4656716
http://www.ncbi.nlm.nih.gov/pubmed/26202488?tool=bestpractice.com
[145]Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017 Jul;5(7):513-23.
http://www.ncbi.nlm.nih.gov/pubmed/28546097?tool=bestpractice.com
[146]Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018 Feb;33(2):190-8.
https://asbmr.onlinelibrary.wiley.com/doi/10.1002/jbmr.3337
http://www.ncbi.nlm.nih.gov/pubmed/29105841?tool=bestpractice.com
Denosumab treatment of postmenopausal osteoporosis for up to 8 years was associated with persistent reduction of bone turnover, continued increase in BMD, low fracture incidence, and high benefit-risk profile
Denosumab treatment for up to 10 years was associated with low rates of adverse events, low incidence of fracture compared with that observed during the original trial, and continued increases in BMD without plateau
Discontinuation of denosumab is followed by rapidly rising turnover markers, decreasing bone density, and increasing vertebral fracture risk, suggesting that patients who discontinue denosumab should transition quickly to an alternative antiresorptive treatment.
There is some evidence to suggest that zoledronic acid given after denosumab is most effective 7-8 months post denosumab discontinuation, and that teriparatide given either prior to or in combination with denosumab increases BMD.[147]Horne AM, Mihov B, Reid IR. Bone loss after romosozumab/denosumab: effects of bisphosphonates. Calcif Tissue Int. 2018 Jul;103(1):55-61.
http://www.ncbi.nlm.nih.gov/pubmed/29445836?tool=bestpractice.com
[148]Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015 Sep 19;386(9999):1147-55.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4620731
http://www.ncbi.nlm.nih.gov/pubmed/26144908?tool=bestpractice.com
[149]Liu Y, Xu Y, Zhang X, et al. Evaluating the clinical efficacy of teriparatide and denosumab combination therapy in postmenopausal osteoporosis: a systematic review and meta-analysis. Altern Ther Health Med. 2024 Jun;30(6):270-5.
https://alternative-therapies.com/oa/index.html
http://www.ncbi.nlm.nih.gov/pubmed/37944971?tool=bestpractice.com
However, further research is needed.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
Denosumab has been reported to be significantly more effective at increasing BMD in postmenopausal women previously treated with bisphosphonates, and has demonstrated significant improvement of BMD in at the lumbar spine, total hip, and femoral neck at 12 and 24 months in patients with low BMD or osteoporosis, compared with bisphosphonates.[150]Zhu Y, Huang Z, Wang Y, et al. The efficacy and safety of denosumab in postmenopausal women with osteoporosis previously treated with bisphosphonates: a review. J Orthop Translat. 2020 May;22:7-13.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7231967
http://www.ncbi.nlm.nih.gov/pubmed/32440494?tool=bestpractice.com
[151]Lyu H, Jundi B, Xu C, et al. Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2019 May 1;104(5):1753-65.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6447951
http://www.ncbi.nlm.nih.gov/pubmed/30535289?tool=bestpractice.com
In patients aged 50 years and older with osteoporosis, denosumab was found to be as effective as zoledronic acid at reducing the risk of fractures.[152]Li W, Ning Z, Yang Z, et al. Safety of denosumab versus zoledronic acid in the older adults with osteoporosis: a meta-analysis of cohort studies. Arch Osteoporos. 2022 Jun 17;17(1):84.
http://www.ncbi.nlm.nih.gov/pubmed/35715524?tool=bestpractice.com
An increased risk of serious infection has been reported in patients treated with denosumab compared with placebo.[153]Diker-Cohen T, Rosenberg D, Avni T, et al. Risk for infections during treatment with denosumab for osteoporosis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020 May 1;105(5):dgz322.
https://academic.oup.com/jcem/article/105/5/1641/5695688
http://www.ncbi.nlm.nih.gov/pubmed/31899506?tool=bestpractice.com
[154]Catton B, Surangiwala S, Towheed T. Is denosumab associated with an increased risk for infection in patients with low bone mineral density? A systematic review and meta-analysis of randomized controlled trials. Int J Rheum Dis. 2021 Jul;24(7):869-79.
http://www.ncbi.nlm.nih.gov/pubmed/33793076?tool=bestpractice.com
However, the overall risk of infection is comparable with other osteoporosis treatments, including bisphosphonates.[153]Diker-Cohen T, Rosenberg D, Avni T, et al. Risk for infections during treatment with denosumab for osteoporosis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2020 May 1;105(5):dgz322.
https://academic.oup.com/jcem/article/105/5/1641/5695688
http://www.ncbi.nlm.nih.gov/pubmed/31899506?tool=bestpractice.com
[154]Catton B, Surangiwala S, Towheed T. Is denosumab associated with an increased risk for infection in patients with low bone mineral density? A systematic review and meta-analysis of randomized controlled trials. Int J Rheum Dis. 2021 Jul;24(7):869-79.
http://www.ncbi.nlm.nih.gov/pubmed/33793076?tool=bestpractice.com
Denosumab has been associated with osteonecrosis of the jaw, impairment of fracture healing, and atypical femoral fractures.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
Denosumab is not associated with increased risk of cardiovascular outcomes compared with placebo or active comparators.[152]Li W, Ning Z, Yang Z, et al. Safety of denosumab versus zoledronic acid in the older adults with osteoporosis: a meta-analysis of cohort studies. Arch Osteoporos. 2022 Jun 17;17(1):84.
http://www.ncbi.nlm.nih.gov/pubmed/35715524?tool=bestpractice.com
[155]Lv F, Cai X, Yang W, et al. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: systematic review and meta-analysis. Bone. 2020 Jan;130:115121.
http://www.ncbi.nlm.nih.gov/pubmed/31678488?tool=bestpractice.com
Anabolic agents
Anabolic agents include teriparatide, abaloparatide, and romosozumab.
Teriparatide and abaloparatide are parathyroid hormone analogues approved to treat osteoporosis in postmenopausal women. Teriparatide is a recombinant form of parathyroid hormone, while abaloparatide is an analogue of human parathyroid hormone-related peptide.
Romosozumab, a monoclonal antibody sclerostin inhibitor that decreases bone resorption and increases bone formation, is approved for the treatment of osteoporosis in postmenopausal women at high risk for fracture (defined as a history of osteoporotic fracture, or multiple risk factors for fracture), or patients who have failed or are intolerant to other available osteoporosis therapy.
Guidelines recommend initial treatment with an anabolic agent for postmenopausal women with a very high risk of fracture, such as those with a very low T-score and a history of severe or multiple vertebral fractures, a recent fracture, or multiple risk factors for fracture.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
[81]Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in post-menopausal women and men >50 years of age. Mar 2024 [internet publication].
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/executive-summary
[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
[113]Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020 Mar 1;105(3):dgaa048.
https://academic.oup.com/jcem/article/105/3/587/5739968
http://www.ncbi.nlm.nih.gov/pubmed/32068863?tool=bestpractice.com
The American College of Obstetrics and Gynecology (ACOG) recommends that teriparatide, abaloparatide, or romosozumab may also be used for those who continue to sustain fractures or have significant bone loss while taking antiresorptive therapy.[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
One meta-analysis found that teriparatide and romosozumab were more effective than oral bisphosphonates for reducing risk of clinical and vertebral fractures regardless of baseline fracture risk indicators (history of previous fractures, age, spine T-score, body mass index [BMI], Fracture Risk Assessment Tool [FRAX] score for major osteoporotic fractures).[156]Händel MN, Cardoso I, von Bülow C, et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ. 2023 May 2;381:e068033.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10152340
http://www.ncbi.nlm.nih.gov/pubmed/37130601?tool=bestpractice.com
However, guidelines suggest initial treatment with an anabolic agent may be most beneficial for patients with a very high risk of fractures in practice, in part because anabolic agents require subcutaneous administration.[81]Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in post-menopausal women and men >50 years of age. Mar 2024 [internet publication].
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/executive-summary
[157]van den Bergh JP, Geusens P, Appelman-Dijkstra NM, et al. The Dutch multidisciplinary guideline osteoporosis and fracture prevention, taking a local guideline to the international arena. Arch Osteoporos. 2024 Apr 2;19(1):23.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10987374
http://www.ncbi.nlm.nih.gov/pubmed/38564062?tool=bestpractice.com
Anabolic agents are not typically used as initial therapy in patients with a high risk of fractures.
The American College of Physicians (ACP) guideline only recommends teriparatide or romosozumab for women with primary osteoporosis and a very high risk of fracture; they concluded that evidence for or against abaloparatide treatment was inconclusive.[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
The ACP suggests that postmenopausal women with prevalent vertebral fractures benefit more from teriparatide treatment than those without prevalent fractures.[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
The Royal Australian College of General Practitioners guideline recommends romosozumab as the preferred first-line option for patients with a very high risk of fracture, based on evidence that it may increase BMD more effectively than alendronic acid or teriparatide.[81]Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in post-menopausal women and men >50 years of age. Mar 2024 [internet publication].
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/executive-summary
Romosozumab:[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
[81]Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in post-menopausal women and men >50 years of age. Mar 2024 [internet publication].
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/executive-summary
[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
[113]Shoback D, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020 Mar 1;105(3):dgaa048.
https://academic.oup.com/jcem/article/105/3/587/5739968
http://www.ncbi.nlm.nih.gov/pubmed/32068863?tool=bestpractice.com
[158]Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016 Oct 20;375(16):1532-43.
https://www.nejm.org/doi/10.1056/NEJMoa1607948
http://www.ncbi.nlm.nih.gov/pubmed/27641143?tool=bestpractice.com
[159]Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017 Oct 12;377(15):1417-27.
https://www.nejm.org/doi/10.1056/NEJMoa1708322
http://www.ncbi.nlm.nih.gov/pubmed/28892457?tool=bestpractice.com
[160]Liu Y, Cao Y, Zhang S, et al. Romosozumab treatment in postmenopausal women with osteoporosis: a meta-analysis of randomized controlled trials. Climacteric. 2018 Apr;21(2):189-95.
http://www.ncbi.nlm.nih.gov/pubmed/29424257?tool=bestpractice.com
[161]Prather C, Adams E, Zentgraf W. Romosozumab: a first-in-class sclerostin inhibitor for osteoporosis. Am J Health Syst Pharm. 2020 Nov 16;77(23):1949-56.
http://www.ncbi.nlm.nih.gov/pubmed/32880646?tool=bestpractice.com
[162]Kaveh S, Hosseinifard H, Ghadimi N, et al. Efficacy and safety of romosozumab in treatment for low bone mineral density: a systematic review and meta-analysis. Clin Rheumatol. 2020 Nov;39(11):3261-76.
http://www.ncbi.nlm.nih.gov/pubmed/32385757?tool=bestpractice.com
[163]Lim SY. Romosozumab for the treatment of osteoporosis in women: efficacy, safety, and cardiovascular risk. Womens Health (Lond). 2022 Jan-Dec;18:17455057221125577.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9511529
http://www.ncbi.nlm.nih.gov/pubmed/36154750?tool=bestpractice.com
[164]Bovijn J, Krebs K, Chen CY, et al. Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics. Sci Transl Med. 2020 Jun 24;12(549):eaay6570.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7116615
http://www.ncbi.nlm.nih.gov/pubmed/32581134?tool=bestpractice.com
[165]Mariscal G, Nuñez JH, Bhatia S, et al. Safety of romosozumab in osteoporotic men and postmenopausal women: a meta-analysis and systematic review. Monoclon Antib Immunodiagn Immunother. 2020 Apr;39(2):29-36.
http://www.ncbi.nlm.nih.gov/pubmed/32195618?tool=bestpractice.com
[166]Nealy KL, Harris KB. Romosozumab: a novel injectable sclerostin inhibitor with anabolic and antiresorptive effects for osteoporosis. Ann Pharmacother. 2021 May;55(5):677-86.
http://www.ncbi.nlm.nih.gov/pubmed/32862655?tool=bestpractice.com
Romosozumab is recommended for the treatment of postmenopausal osteoporosis for up to 1 year. Romosozumab should be avoided in patients with a high risk for cardiovascular disease or stroke.
Treatment with romosozumab should be followed by sequential therapy with an antiresorptive agent to maintain BMD gains and reduce the risk of fracture.
Romosozumab has demonstrated significant reductions in new vertebral fractures, new non-vertebral fractures, and hip fracture compared with other osteoporosis therapies, and an increase in BMD at lumbar spine, total hip, and femoral neck compared with placebo in postmenopausal women and other patients with osteoporosis and low BMD.
A subsequent systematic review reported that romosozumab increased BMD at the lumbar spine and total hip compared with teriparatide at 12 months in patients with osteoporosis. In postmenopausal women at a higher risk of fracture, 1 year of romosozumab followed by 1 year of alendronic acid reduced the risk of vertebral, non-vertebral, clinical, and hip fractures compared with alendronic acid alone at 24 months. Cardiovascular and cerebrovascular events were higher in the romosozumab group compared with alendronic acid.
Some evidence suggests that romosozumab may have a lower rate of adverse effects compared with alendronic acid and a similar safety profile to bisphosphonates; however, most studies agree that further research is needed to establish the risk of cardiovascular disease with romosozumab treatment.
Teriparatide and abaloparatide:[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
[167]Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001 May 10;344(19):1434-41.
http://www.nejm.org/doi/full/10.1056/NEJM200105103441904
http://www.ncbi.nlm.nih.gov/pubmed/11346808?tool=bestpractice.com
[168]Yang C, Le G, Lu C, et al. Effects of teriparatide compared with risedronate in the treatment of osteoporosis: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020 Feb;99(7):e19042.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7035098
http://www.ncbi.nlm.nih.gov/pubmed/32049802?tool=bestpractice.com
[169]Shen J, Ke Z, Dong S, et al. Pharmacological therapies for osteoporosis: a Bayesian network meta-analysis. Med Sci Monit. 2022 Apr 17;28:e935491.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9022483
http://www.ncbi.nlm.nih.gov/pubmed/35430576?tool=bestpractice.com
[170]Tian A, Jia H, Zhu S, et al. Romosozumab versus teriparatide for the treatment of postmenopausal osteoporosis: a systematic review and meta-analysis through a grade analysis of evidence. Orthop Surg. 2021 Oct;13(7):1941-50.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8528978
http://www.ncbi.nlm.nih.gov/pubmed/34643048?tool=bestpractice.com
[171]Poutoglidou F, Samoladas E, Raikos N, et al. Efficacy and safety of anti-sclerostin antibodies in the treatment of osteoporosis: a meta-analysis and systematic review. J Clin Densitom. 2022 Jul-Sep;25(3):401-15.
http://www.ncbi.nlm.nih.gov/pubmed/34920938?tool=bestpractice.com
[172]Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016 Aug 16;316(7):722-33.
http://www.ncbi.nlm.nih.gov/pubmed/27533157?tool=bestpractice.com
[173]Cosman F, Miller PD, Williams GC, et al. Eighteen months of treatment with subcutaneous abaloparatide followed by 6 months of treatment with alendronate in postmenopausal women with osteoporosis: results of the ACTIVExtend trial. Mayo Clin Proc. 2017 Feb;92(2):200-10.
https://www.mayoclinicproceedings.org/article/S0025-6196(16)30630-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28160873?tool=bestpractice.com
[174]Bone HG, Cosman F, Miller PD, et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018 Aug 1;103(8):2949-57.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6097601
http://www.ncbi.nlm.nih.gov/pubmed/29800372?tool=bestpractice.com
[175]Miller PD, Bilezikian JP, Fitzpatrick LA, et al. Abaloparatide: an anabolic treatment to reduce fracture risk in postmenopausal women with osteoporosis. Curr Med Res Opin. 2020 Nov;36(11):1861-72.
https://www.tandfonline.com/doi/full/10.1080/03007995.2020.1824897
http://www.ncbi.nlm.nih.gov/pubmed/32969719?tool=bestpractice.com
[176]Reginster J-Y, Bianic F, Campbell R, et al. Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporos Int. 2019 Jul;30(7):1465-73.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6614166
http://www.ncbi.nlm.nih.gov/pubmed/30953114?tool=bestpractice.com
Guidelines recommend treatment with teriparatide or abaloparatide for up to 2 years. Teriparatide treatment for more than 2 years can be considered if the patient remains or returns to having a high risk of fracture.
The Bone Health and Osteoporosis Foundation (BHOF) suggests that teriparatide and abaloparatide should be avoided in patients at an increased risk of osteosarcoma (Paget's disease of the bone, prior radiotherapy involving the skeleton, history of bone metastases or malignancies, unexplained elevated alkaline phosphatase, and hereditary disorders predisposing to osteosarcoma).
When treatment is stopped, bone loss can be rapid and alternative agents should be considered to maintain BMD. The BHOF and the Endocrine Society recommend that teriparatide or abaloparatide treatment is followed with an antiresorptive agent, usually a bisphosphonate, to maintain or further increase BMD.
Clinical trials have demonstrated that teriparatide increases BMD in comparison with placebo. Evidence of the effectiveness of teriparatide in comparison with other treatments for osteoporosis varies. One systematic review demonstrated that teriparatide improves BMD in lumbar spine, femoral neck, and total hip, and reduces the incidence of clinical fracture, new vertebral fracture, and non-vertebral fracture compared with risedronate. Conversely, a network meta-analysis suggests that teriparatide is less effective compared with other drugs such as bisphosphonates, denosumab, or romosozumab at reducing fracture risk in patients with osteoporosis. Two further systematic reviews demonstrated that teriparatide was less effective than romosozumab at improving BMD of lumbar spine, total hip, and femoral neck for patients with postmenopausal osteoporosis at 6 and 12 months.
The ACTIVE phase 3 double-blind controlled trial and subsequent extension trials demonstrated that abaloparatide reduced the risk of new vertebral and non-vertebral fractures over 18 months compared with placebo, and that sequential use of alendronic acid for 6 or 24 months post abaloparatide treatment reduced the risk of vertebral, non-vertebral, clinical, and major osteoporotic fractures and increased BMD compared with placebo in postmenopausal women.
Results from network meta-analyses indicate that abaloparatide reduces the risk of vertebral fracture, non-vertebral fracture, and wrist fracture compared with other treatments for osteoporosis such as teriparatide, romosozumab, or ibandronic acid in patients with osteoporosis, including postmenopausal women.
Adverse effects of teriparatide include leg cramps, nausea, and dizziness, and for abaloparatide they include nausea, postural hypotension, dizziness, headache, and palpitations.
SERMs
SERMs include raloxifene and bazedoxifene.
Raloxifene is approved for the treatment and prevention of osteoporosis in postmenopausal women. It increases BMD at the spine but to a lesser degree than bisphosphonates, and is only effective in lowering vertebral fracture.[125]Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a
randomized trial. JAMA. 2006 Dec 27;296(24):2927-38.
http://jama.jamanetwork.com/article.aspx?articleid=204789
http://www.ncbi.nlm.nih.gov/pubmed/17190893?tool=bestpractice.com
Raloxifene reduces the risk of vertebral fractures in postmenopausal women with osteoporosis.[177]Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999 Aug 18;282(7):637-45.
https://jamanetwork.com/journals/jama/fullarticle/191242
http://www.ncbi.nlm.nih.gov/pubmed/10517716?tool=bestpractice.com
There is no evidence for reduction of non-vertebral fractures.
Raloxifene use is associated with an increased risk of venous thrombosis and stroke.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
Possibility of adverse effects is weighed against potential benefits of reduced risk of vertebral fracture and oestrogen receptor-positive breast cancer.
The ACOG suggests raloxifene for postmenopausal patients at increased risk of vertebral fracture and breast cancer who are at low risk of venous thromboembolism and do not have significant vasomotor symptoms.[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
The Endocrine Society recommends raloxifene or bazedoxifene to reduce the risk of vertebral fracture in postmenopausal women aged under 60 years or <10 years past menopause, with osteoporosis at high risk of fracture and who have a low risk of deep vein thrombosis, and for whom bisphosphonates or denosumab are not appropriate, or have a high risk of breast cancer.[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
The ACP concluded that there is insufficient evidence to recommend for or against treatment with raloxifene or bazedoxifene.[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
One systematic review demonstrated that raloxifene is effective at improving lumbar spine BMD in postmenopausal women with end-stage renal disease compared with placebo, with a mean duration of treatment of 12 months.[178]Ma HY, Chen S, Lu LL, et al. Raloxifene in the treatment of osteoporosis in postmenopausal women with end-stage renal disease: a systematic review and meta-analysis. Horm Metab Res. 2021 Nov;53(11):730-7.
https://www.thieme-connect.com/products/ejournals/html/10.1055/a-1655-4362
http://www.ncbi.nlm.nih.gov/pubmed/34740274?tool=bestpractice.com
No adverse effects were reported in the raloxifene patient group, but further large RCTs are needed to evaluate the long-term safety of raloxifene in these patients.[178]Ma HY, Chen S, Lu LL, et al. Raloxifene in the treatment of osteoporosis in postmenopausal women with end-stage renal disease: a systematic review and meta-analysis. Horm Metab Res. 2021 Nov;53(11):730-7.
https://www.thieme-connect.com/products/ejournals/html/10.1055/a-1655-4362
http://www.ncbi.nlm.nih.gov/pubmed/34740274?tool=bestpractice.com
Bazedoxifene reduces the risk of endometrial hyperplasia (excessive growth of the lining of the uterus) that can occur with oestrogen treatment.[179]Pinkerton JV, Thomas S, Dalkin AC. Osteoporosis treatment and prevention for postmenopausal women: current and future therapeutic options. Clin Obstet Gynecol. 2013 Dec;56(4):711-21.
http://www.ncbi.nlm.nih.gov/pubmed/24100598?tool=bestpractice.com
Bazedoxifene is only available in a combination formulation with conjugated oestrogens in the US; however, it is available as a single-ingredient formulation in other countries. Conjugated oestrogens/bazedoxifene is recommended third-line only after careful consideration of alternative agents that do not contain oestrogen (see below).[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
Hormone therapy
Oestrogen decline at menopause is strongly associated with the decrease in BMD.[2]NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001 Feb 14;285(6):785-95.
http://www.ncbi.nlm.nih.gov/pubmed/11176917?tool=bestpractice.com
[3]WHO Scientific Group on the Prevention and Management of Osteoporosis. Prevention and management of osteoporosis: report of a WHO scientific group. (WHO technical report series: 921.) Geneva, Switzerland: WHO; 2003.
https://apps.who.int/iris/handle/10665/42841
There are various forms of hormone replacement therapy (HRT). Oestrogen, either alone or in combination with a progestin, is considered only for women at high risk of osteoporotic fractures for whom non-hormonal therapy is inappropriate.
HRT reduces the incidence of fracture. However, there are considerable increases in risk of coronary heart disease, breast cancer, venous thrombosis, and stroke.[180]Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010 Jul;95(7 suppl 1):s1-66.
https://academic.oup.com/jcem/article/95/7_supplement_1/s1/2835479
http://www.ncbi.nlm.nih.gov/pubmed/20566620?tool=bestpractice.com
[181]Medicines and Healthcare products Regulatory Agency. Hormone replacement therapy (HRT): further information on the known increased risk of breast cancer with HRT and its persistence after stopping. Aug 2019 [internet publication].
https://www.gov.uk/drug-safety-update/hormone-replacement-therapy-hrt-further-information-on-the-known-increased-risk-of-breast-cancer-with-hrt-and-its-persistence-after-stopping
[182]Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019 Sep 28;394(10204):1159-68.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31709-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/31474332?tool=bestpractice.com
The WHI report assessed the efficacy of conjugated oestrogens versus placebo in 10,739 postmenopausal women, aged 50-79 years with prior hysterectomy.[183]Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701-12.
http://www.ncbi.nlm.nih.gov/pubmed/15082697?tool=bestpractice.com
The primary outcome was coronary heart disease. The study reported that conjugated oestrogens increased the risk of stroke, decreased the risk of hip fracture, and did not affect the incidence of coronary heart disease in this population at an average of 6.8 years.[183]Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701-12.
http://www.ncbi.nlm.nih.gov/pubmed/15082697?tool=bestpractice.com
The Endocrine Society recommends oestrogen as first-line treatment to prevent all types of fracture for women (under 60 years of age or <10 years past menopause) with hysterectomy at high risk of osteoporotic fracture, with a low risk of deep vein thrombosis, for whom bisphosphonates or denosumab are not appropriate, who have vasomotor symptoms, who have no contraindications, no prior myocardial infarction, stroke, or breast cancer, and are willing to take menopausal hormone therapy.[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
Women who have a uterus should use oestrogen only in combination with a progestin because using oestrogen alone increases the incidence of endometrial cancer.[184]Sjögren LL, Mørch LS, Løkkegaard E. Hormone replacement therapy and the risk of endometrial cancer: a systematic review. Maturitas. 2016 Sep;91:25-35.
http://www.ncbi.nlm.nih.gov/pubmed/27451318?tool=bestpractice.com
The Endocrine Society recommends oestrogen plus a progestin or tibolone (not available in the US) as first-line treatment to prevent vertebral and non-vertebral fractures for women aged under 60 years (or <10 years past menopause) at high risk of osteoporotic fracture, with a low risk of deep vein thrombosis, for whom bisphosphonates or denosumab are not appropriate, who have vasomotor symptoms, who have no contraindications, no prior myocardial infarction, stroke, or breast cancer, and are willing to take menopausal tibolone therapy.[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
The Endocrine Society also recommends oestrogen plus a progestin or tibolone as second-line treatment to prevent vertebral and non-vertebral fractures for women aged over 60 years for whom bisphosphonates, denosumab, and teriparatide/abaloparatide are not appropriate.[108]Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104(5):1595-622.
https://www.endocrine.org/clinical-practice-guidelines/osteoporosis-in-postmenopausal-women
http://www.ncbi.nlm.nih.gov/pubmed/30907953?tool=bestpractice.com
The ACP recommends against using menopausal oestrogen alone or in combination with progestin therapy for the treatment of osteoporosis in women.[185]Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10885682
http://www.ncbi.nlm.nih.gov/pubmed/28492856?tool=bestpractice.com
Conjugated oestrogens/bazedoxifene
Conjugated oestrogens/bazedoxifene are indicated for osteoporosis prophylaxis due to menopause in women with an intact uterus.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
[70]ACOG Committee on Clinical Practice Guidelines–Gynecology. Management of postmenopausal osteoporosis: ACOG clinical practice guideline no. 2. Obstet Gynecol. 2022 Apr 1;139(4):698-717.
http://www.ncbi.nlm.nih.gov/pubmed/35594133?tool=bestpractice.com
Bazedoxifene on its own is indicated for the treatment of postmenopausal osteoporosis in women at increased risk of fracture, but it is not available as a single-ingredient formulation in the US.
The BHOF recommends conjugated oestrogens/bazedoxifene for the prevention of osteoporosis only in women at significant risk of fracture and only after careful consideration of alternative treatments that do not contain oestrogen.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
This treatment should only be used for the shortest duration consistent with treatment goals and risks for the individual woman.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
Adverse effects include muscle spasms, nausea, diarrhoea, dyspepsia, upper abdominal pain, oropharyngeal pain, dizziness, and neck pain.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
Men
Men aged 50 years and older presenting with any of the following should be considered for osteoporosis treatment:[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
A hip or vertebral fracture (clinically apparent or found on vertebral imaging) regardless of T-score
A fracture of the pelvis, proximal humerus, or distal forearm in a person with low bone mass or osteopenia
T-score ≤-2.5 at the femoral neck, total hip, lumbar spine, or 33% radius
High fracture risk and need for pharmacological intervention as indicated by T-score between -1.0 and -2.5 at the femoral neck or total hip and a 10-year probability of a hip fracture ≥3% or a 10-year probability of a major osteoporosis-related fracture ≥20% (based on FRAX).
Bisphosphonates
Oral bisphosphonates are the preferred first-line treatment for men with osteoporosis and a high risk of fracture, with or without prior vertebral fracture.[82]Fuggle NR, Beaudart C, Bruyère O, et al. Evidence-based guideline for the management of osteoporosis in men. Nat Rev Rheumatol. 2024 Apr;20(4):241-51.
https://www.nature.com/articles/s41584-024-01094-9
http://www.ncbi.nlm.nih.gov/pubmed/38485753?tool=bestpractice.com
Alendronic acid, risedronate, and zoledronic acid are approved for the treatment of osteoporosis in men. However, despite significant understanding of the pathogenesis and management of male osteoporosis, several key issues remain unresolved. In the US, the FDA has not approved the use of zoledronic acid for men with osteoporosis and testosterone deficiency.
A multicentre, double-blind, placebo-controlled trial of men with primary and hypogonadism-associated osteoporosis aged 50-85 years found that an intravenous infusion of zoledronic acid at baseline and 12 months reduces the risk of new morphometric vertebral fracture by 67% over a 24-month period compared with placebo.[186]Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012 Nov 1;367(18):1714-23.
http://www.nejm.org/doi/full/10.1056/NEJMoa1204061
http://www.ncbi.nlm.nih.gov/pubmed/23113482?tool=bestpractice.com
The ACP guideline indicates that the response to bisphosphonate and denosumab treatment is similar in men as well as in women.[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
Adverse effects of oral bisphosphonates primarily relate to the upper gastrointestinal tract and include difficulty swallowing, oesophagitis, and gastric ulcers. Joint and muscular pain, osteonecrosis of the jaw, and atypical femoral fractures have also been reported.[117]Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014 Jan;29(1):1-23.
https://onlinelibrary.wiley.com/doi/full/10.1002/jbmr.1998
http://www.ncbi.nlm.nih.gov/pubmed/23712442?tool=bestpractice.com
[118]Abrahamsen B. Adverse effects of bisphosphonates. Calcif Tissue Int. 2010 Jun;86(6):421-35.
http://www.ncbi.nlm.nih.gov/pubmed/20407762?tool=bestpractice.com
[119]Lee S, Yin RV, Hirpara H, et al. Increased risk for atypical fractures associated with bisphosphonate use. Fam Pract. 2015 Jun;32(3):276-81.
http://fampra.oxfordjournals.org/content/32/3/276.long
http://www.ncbi.nlm.nih.gov/pubmed/25846215?tool=bestpractice.com
[120]Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint and muscle pain. Arch Intern Med. 2005 Feb 14;165(3):346-7.
http://www.ncbi.nlm.nih.gov/pubmed/15710802?tool=bestpractice.com
[121]Khan AA, Sándor GK, Dore E, et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009 Mar;36(3):478-90.
http://www.ncbi.nlm.nih.gov/pubmed/19286860?tool=bestpractice.com
[122]Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006 May 16;144(10):753-61.
http://www.ncbi.nlm.nih.gov/pubmed/16702591?tool=bestpractice.com
Testosterone
Testosterone deficiency has been associated with decreased BMD in men, but evidence that testosterone replacement therapy improves BMD or reduces the risk of fractures is limited and inconsistent.[82]Fuggle NR, Beaudart C, Bruyère O, et al. Evidence-based guideline for the management of osteoporosis in men. Nat Rev Rheumatol. 2024 Apr;20(4):241-51.
https://www.nature.com/articles/s41584-024-01094-9
http://www.ncbi.nlm.nih.gov/pubmed/38485753?tool=bestpractice.com
One systematic review concluded that testosterone therapy did not increase BMD in the spine, the femoral neck, Ward’s triangle, and the whole body, with the exception of the trochanter and total hip in older men.[187]Junjie W, Dongsheng H, Lei S, et al. Testosterone replacement therapy has limited effect on increasing bone mass density in older men: a meta-analysis. Curr Pharm Des. 2019;25(1):73-84.
http://www.ncbi.nlm.nih.gov/pubmed/30727867?tool=bestpractice.com
Testosterone therapy may be considered as an adjunct to osteoporosis-specific therapy in patients with symptomatic testosterone deficiency.[82]Fuggle NR, Beaudart C, Bruyère O, et al. Evidence-based guideline for the management of osteoporosis in men. Nat Rev Rheumatol. 2024 Apr;20(4):241-51.
https://www.nature.com/articles/s41584-024-01094-9
http://www.ncbi.nlm.nih.gov/pubmed/38485753?tool=bestpractice.com
[188]Jayasena CN, Anderson RA, Llahana S, et al. Society for endocrinology guidelines for testosterone replacement therapy in male hypogonadism. Clin Endocrinol (Oxf). 2022 Feb;96(2):200-19.
https://onlinelibrary.wiley.com/doi/epdf/10.1111/cen.14633
http://www.ncbi.nlm.nih.gov/pubmed/34811785?tool=bestpractice.com
It is not recommended for men with normal testosterone levels.
Anabolic agents
Some guidelines recommend considering initial treatment with an anabolic agent (e.g., teriparatide, abaloparatide, romosozumab) for men with a very high risk of fractures.[81]Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in post-menopausal women and men >50 years of age. Mar 2024 [internet publication].
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/executive-summary
[82]Fuggle NR, Beaudart C, Bruyère O, et al. Evidence-based guideline for the management of osteoporosis in men. Nat Rev Rheumatol. 2024 Apr;20(4):241-51.
https://www.nature.com/articles/s41584-024-01094-9
http://www.ncbi.nlm.nih.gov/pubmed/38485753?tool=bestpractice.com
This should be followed by sequential therapy with an antiresorptive agent.[82]Fuggle NR, Beaudart C, Bruyère O, et al. Evidence-based guideline for the management of osteoporosis in men. Nat Rev Rheumatol. 2024 Apr;20(4):241-51.
https://www.nature.com/articles/s41584-024-01094-9
http://www.ncbi.nlm.nih.gov/pubmed/38485753?tool=bestpractice.com
However, although the ACP guideline recommends this approach for women with a very high risk of fractures, they concluded that evidence was insufficient to make a similar recommendation for men.[67]Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. 2023 Feb;176(2):224-38.
https://www.acpjournals.org/doi/full/10.7326/M22-1034
http://www.ncbi.nlm.nih.gov/pubmed/36592456?tool=bestpractice.com
Guidelines suggest initial treatment with an anabolic agent may be most beneficial for patients with a very high risk of fractures in practice, and they are not typically used as initial therapy in patients with a high risk of fractures.[81]Royal Australian College of General Practitioners. Osteoporosis management and fracture prevention in post-menopausal women and men >50 years of age. Mar 2024 [internet publication].
https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/osteoporosis/executive-summary
[99]Morin SN, Feldman S, Funnell L, et al. Clinical practice guideline for management of osteoporosis and fracture prevention in Canada: 2023 update. CMAJ. 2023 Oct 10;195(39):E1333-48.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10610956
http://www.ncbi.nlm.nih.gov/pubmed/37816527?tool=bestpractice.com
[157]van den Bergh JP, Geusens P, Appelman-Dijkstra NM, et al. The Dutch multidisciplinary guideline osteoporosis and fracture prevention, taking a local guideline to the international arena. Arch Osteoporos. 2024 Apr 2;19(1):23.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10987374
http://www.ncbi.nlm.nih.gov/pubmed/38564062?tool=bestpractice.com
Teriparatide and abaloparatide are approved for men with a high fracture risk.[189]Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008 Jun;29(4):441-64.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2528848
http://www.ncbi.nlm.nih.gov/pubmed/18451258?tool=bestpractice.com
Abaloparatide significantly increases BMD at the lumbar spine, total hip, and femoral neck compared with placebo in men with osteoporosis.[190]Czerwinski E, Cardona J, Plebanski R, et al. The efficacy and safety of abaloparatide-SC in men with osteoporosis: a randomized clinical trial. J Bone Miner Res. 2022 Dec;37(12):2435-42.
https://asbmr.onlinelibrary.wiley.com/doi/10.1002/jbmr.4719
http://www.ncbi.nlm.nih.gov/pubmed/36190391?tool=bestpractice.com
[191]Beaudart C, Demonceau C, Sabico S, et al. Efficacy of osteoporosis pharmacological treatments in men: a systematic review and meta-analysis. Aging Clin Exp Res. 2023 Sep;35(9):1789-806.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10460304
http://www.ncbi.nlm.nih.gov/pubmed/37400668?tool=bestpractice.com
European guidelines for osteoporosis in men concluded that evidence of BMD improvement is strongest for abaloparatide.[82]Fuggle NR, Beaudart C, Bruyère O, et al. Evidence-based guideline for the management of osteoporosis in men. Nat Rev Rheumatol. 2024 Apr;20(4):241-51.
https://www.nature.com/articles/s41584-024-01094-9
http://www.ncbi.nlm.nih.gov/pubmed/38485753?tool=bestpractice.com
In the UK, teriparatide is recommended as an alternative treatment option for the secondary prevention of osteoporotic fractures in men.[192]NHS England. Interim clinical commissioning policy statement: teriparatide for osteoporosis in men (adults). Jan 2021 [internet publication].
https://www.england.nhs.uk/publication/interim-clinical-commissioning-policy-statement-teriparatide-for-osteoporosis-in-men-adults-2
RCTs in men with osteoporosis report that teriparatide increases BMD at the spine and femoral neck.[193]Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003 Jan;18(1):9-17.
https://asbmr.onlinelibrary.wiley.com/doi/full/10.1359/jbmr.2003.18.1.9
http://www.ncbi.nlm.nih.gov/pubmed/12510800?tool=bestpractice.com
Although BMD gradually decreases after discontinuation of treatment, when followed by antiresorptive treatment, teriparatide decreases the risk of moderate and severe vertebral fracture.[194]Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005 May;16(5):510-6.
http://www.ncbi.nlm.nih.gov/pubmed/15322742?tool=bestpractice.com
Evidence also suggests that teriparatide is as effective in men as in postmenopausal women to treat osteoporosis.[195]Niimi R, Kono T, Nishihara A, et al. Analysis of daily teriparatide treatment for osteoporosis in men. Osteoporos Int. 2015 Apr;26(4):1303-9.
http://www.ncbi.nlm.nih.gov/pubmed/25567777?tool=bestpractice.com
When treatment with teriparatide or abaloparatide is stopped, bone loss can be rapid and alternative agents should be considered to maintain bone mineral density.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
[82]Fuggle NR, Beaudart C, Bruyère O, et al. Evidence-based guideline for the management of osteoporosis in men. Nat Rev Rheumatol. 2024 Apr;20(4):241-51.
https://www.nature.com/articles/s41584-024-01094-9
http://www.ncbi.nlm.nih.gov/pubmed/38485753?tool=bestpractice.com
Romosozumab is not approved for use in men with osteoporosis in the US or Europe, but it is approved in some other countries to treat men with osteoporosis at high risk of fracture.[62]LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022 Oct;33(10):2049-102.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9546973
http://www.ncbi.nlm.nih.gov/pubmed/35478046?tool=bestpractice.com
Romosozumab treatment for 12 months resulted in significantly higher BMD at the spine, femoral neck, and total hip compared with placebo, and was well tolerated in men with osteoporosis.[196]Lewiecki EM, Blicharski T, Goemaere S, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018 Sep 1;103(9):3183-93.
https://academic.oup.com/jcem/article/103/9/3183/5040365
http://www.ncbi.nlm.nih.gov/pubmed/29931216?tool=bestpractice.com
Denosumab
Denosumab is approved for the treatment of men with osteoporosis who are at high risk of fractures, defined as a history of fragility factors, or multiple risk factors for fractures; or those who failed or are intolerant to other available osteoporosis drug regimens (see the FDA warning in the information on denosumab in the postmenopausal women section).
It is also approved for osteoporosis prophylaxis in men at high risk for fracture after receiving androgen deprivation therapy for non-metastatic prostate cancer. In men with non-metastatic prostate cancer, denosumab also reduced the incidence of vertebral fracture.[197]Smith MR, Egerdie B, Hernández Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009 Aug 20;361(8):745-55.
https://www.nejm.org/doi/full/10.1056/NEJMoa0809003
http://www.ncbi.nlm.nih.gov/pubmed/19671656?tool=bestpractice.com
Glucocorticoid-induced osteoporosis
Corticosteroid-induced osteoporosis is a complex disorder that encompasses both increased bone resorption and defective bone formation. Bone fracture occurs in 30% to 50% of patients receiving chronic corticosteroid treatment; therefore, its prevention and treatment is imperative in this population.[65]Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015 Apr 8;1(1):e000014.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4613168
http://www.ncbi.nlm.nih.gov/pubmed/26509049?tool=bestpractice.com
Patients receiving long-term corticosteroids should be assessed for fracture risk at initiation of therapy and every 1-2 years while corticosteroid treatment continues.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Treatment and prevention of corticosteroid induced osteoporosis is based on age and risk of fracture, ranging from low to very high risk. See Diagnostic criteria.
[Figure caption and citation for the preceding image starts]: Management of glucocorticoid-induced osteoporosisCreated by BMJ Knowledge Centre [Citation ends].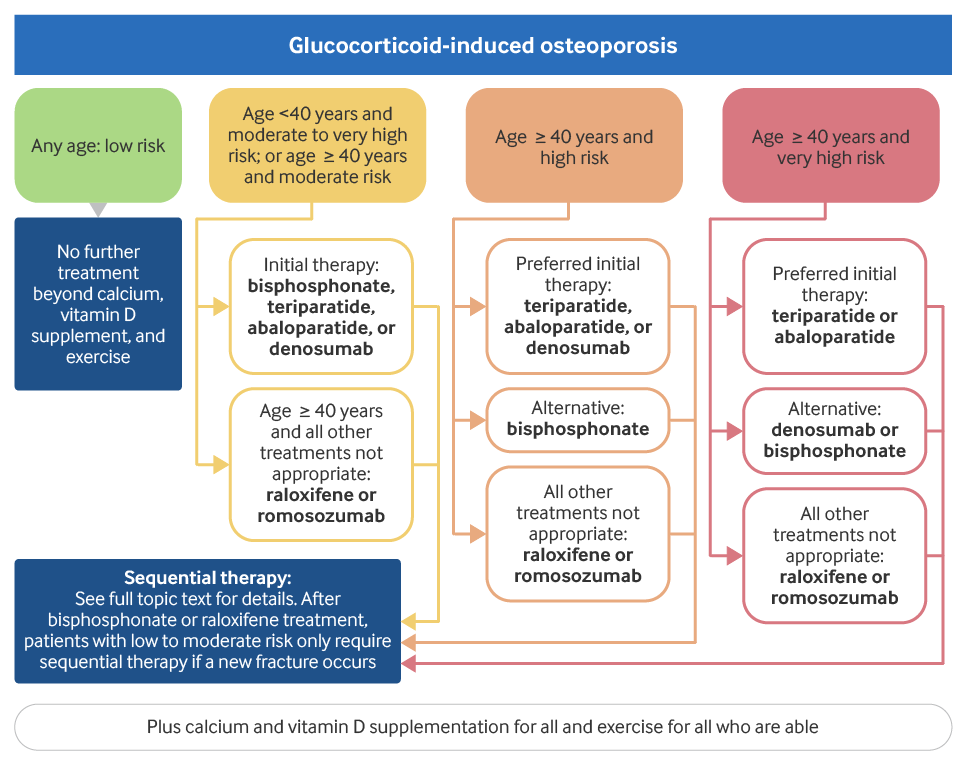
Lifestyle changes and supplements
The American College of Rheumatology (ACR) recommends all adults taking prednisolone at a dose of ≥2.5 mg/day for ≥3 months should optimise calcium and vitamin D intake (with supplementation if needed) and make lifestyle modifications (e.g., balanced diet, maintaining weight in the recommended range, smoking cessation, regular weight‐bearing or resistance training exercise, limiting alcohol intake to 1-2 alcoholic beverages per day).[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
These measures should be used in combination with pharmacological interventions for patients with moderate to very high risk of fracture, however no further treatment is recommended for patients with low fracture risk.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Bisphosphonates
Bisphosphonate use should be considered for most patients with corticosteroid treatment continuing >3 months, receiving from 2.5 to ≥7.5 mg/day of prednisolone, and in those with a history of prior fracture.[24]Gregson CL, Armstrong DJ, Bowden J, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2022 Apr 5;17(1):58.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8979902
http://www.ncbi.nlm.nih.gov/pubmed/35378630?tool=bestpractice.com
[66]Hsu E, Nanes M. Advances in treatment of glucocorticoid-induced osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2017 Dec;24(6):411-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5836323
http://www.ncbi.nlm.nih.gov/pubmed/28857847?tool=bestpractice.com
The ACR recommends oral or intravenous bisphosphonates for adults at moderate, high risk, or very high risk of fracture who are receiving long-term corticosteroids.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
For adults aged ≥40 years at high or very high risk of fracture, bisphosphonates are conditionally recommended second-line based on evidence of superior BMD improvements with parathyroid hormone analogues and denosumab.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Patients with chronic kidney disease (eGFR <35 mL/minute) should generally not be treated with bisphosphonates.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
For women of childbearing age who are not planning a pregnancy during osteoporosis treatment, an oral or intravenous bisphosphonate is recommended, but should be used with caution due to potential adverse effects to fetal bones.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Patients treated with oral or intravenous bisphosphonates who have a low to moderate risk of fracture and discontinue corticosteroid treatment do not require sequential therapy.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
However, if a new fracture occurs after ≥12 months of initial bisphosphonate therapy, sequential therapy may include denosumab, parathyroid hormone analogue or romosozumab.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
If treated with denosumab sequential therapy, patients will require additional therapy with bisphosphonates 6-7 months after the last dose of denosumab to prevent rapid bone loss and development of new vertebral compression fractures.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
The bisphosphonates alendronic acid and risedronate have been shown to effectively reduce bone fracture for patients with corticosteroid induced osteoporosis.[198]van Brussel MS, Bultink IE, Lems WF. Prevention of glucocorticoid-induced osteoporosis. Expert Opin Pharmacother. 2009 Apr;10(6):997-1005.
http://www.ncbi.nlm.nih.gov/pubmed/19351276?tool=bestpractice.com
[  ]
How do bisphosphonates compare with placebo for improving outcomes in people with steroid-induced osteoporosis?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1617/fullShow me the answer[Evidence A]afadd294-f705-412a-b6d4-04a1a35a927cccaAHow do bisphosphonates compare with placebo for improving outcomes in people with corticosteroid‐induced osteoporosis?
]
How do bisphosphonates compare with placebo for improving outcomes in people with steroid-induced osteoporosis?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1617/fullShow me the answer[Evidence A]afadd294-f705-412a-b6d4-04a1a35a927cccaAHow do bisphosphonates compare with placebo for improving outcomes in people with corticosteroid‐induced osteoporosis?
Parathyroid hormone analogues
The ACR recommend a parathyroid hormone analogue (e.g., teriparatide, abaloparatide) as a first-line option for the treatment of adults ≥40 years with high to very high risk of fracture, including patients on very high-dose corticosteroid treatment (initial prednisolone dose ≥30 mg/day or cumulative prednisolone dose ≥5 g in 1 year).[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
For patients with a moderate risk of fracture, a parathyroid hormone analogue may be considered as an alternative option.[24]Gregson CL, Armstrong DJ, Bowden J, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2022 Apr 5;17(1):58.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8979902
http://www.ncbi.nlm.nih.gov/pubmed/35378630?tool=bestpractice.com
[199]Anastasilaki E, Paccou J, Gkastaris K, et al. Glucocorticoid-induced osteoporosis: an overview with focus on its prevention and management. Hormones (Athens). 2023 Dec;22(4):611-22.
http://www.ncbi.nlm.nih.gov/pubmed/37755658?tool=bestpractice.com
The ACR recommends them as an equal first-line option for adults with a moderate risk of fracture, and emphasises shared decision-making for choice of initial therapy, considering clinician and patient preferences and comorbidities.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Parathyroid hormone analogues should be avoided for the treatment of young adults with open growth plates.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Teriparatide treatment has been demonstrated to increase BMD of the lumbar vertebrae compared with denosumab, and reduces the risk of vertebral fracture, compared with bisphosphonates, for patients with corticosteroid induced osteoporosis.[200]Yuan C, Liang Y, Zhu K, et al. Clinical efficacy of denosumab, teriparatide, and oral bisphosphonates in the prevention of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. J Orthop Surg Res. 2023 Jun 22;18(1):447.
https://josr-online.biomedcentral.com/articles/10.1186/s13018-023-03920-4
http://www.ncbi.nlm.nih.gov/pubmed/37349750?tool=bestpractice.com
Pre-clinical and animal trials suggest that abaloparatide may mitigate or prevent bone loss from glucocorticoids and improve fracture healing.[201]Brent MB. Abaloparatide: a review of preclinical and clinical studies. Eur J Pharmacol. 2021 Oct 15;909:174409.
https://www.doi.org/10.1016/j.ejphar.2021.174409
http://www.ncbi.nlm.nih.gov/pubmed/34364879?tool=bestpractice.com
There are no available data on the evaluation of abaloparatide for the treatment of corticosteroid-induced osteoporosis from clinical trials.
Patients should be advised that stopping parathyroid hormone analogue treatment without transition to another therapy can result in bone loss. This can be prevented by starting oral or intravenous bisphosphonates for patients at low to moderate risk of fracture when the parathyroid hormone analogue and corticosteroids are discontinued.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
If a fracture occurs when the patient has been treated with a parathyroid hormone analogue for ≥12 months, sequential therapy may include oral or intravenous bisphosphonates or denosumab.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
If treated with denosumab sequential therapy, patients will require additional therapy with bisphosphonates 6-7 months after the last dose of denosumab to prevent rapid bone loss and development of new vertebral compression fractures.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Denosumab
Denosumab is approved for the management of glucocorticoid-induced osteoporosis in both men and women with a high risk of fracture, including those with a history of osteoporotic fractures, multiple clinical risk factors for fracture, and those who cannot tolerate or have not responded to other treatments (see the FDA warning in the information on denosumab in the postmenopausal women section).
The ACR recommends denosumab as a first-line option for adults with a moderate risk of fracture and adults aged ≥40 years at high risk of fracture.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
For adults aged ≥40 years at very high risk of fractures, denosumab is recommended as a second-line option.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Denosumab should be used with caution in women of child bearing age due to potential adverse effects for the fetus, pregnancy should be avoided until 5 months after the last dose.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Denosumab treatment should be avoided when treating young adults with open growth plates.
One systematic review reported that denosumab significantly increased BMD in the lumbar spine at 6 months, and in the lumbar spine and femoral neck at 12 months, compared with bisphosphonate therapy in patients with corticosteroid-induced osteoporosis.[202]Yamaguchi Y, Morita T, Kumanogoh A. The therapeutic efficacy of denosumab for the loss of bone mineral density in glucocorticoid-induced osteoporosis: a meta-analysis. Rheumatol Adv Pract. 2020;4(1):rkaa008.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7197806
http://www.ncbi.nlm.nih.gov/pubmed/32373775?tool=bestpractice.com
Patients with corticosteroid-induced osteoporosis treated with denosumab with a low to moderate risk of fracture when denosumab and corticosteroid therapy are stopped should receive 1-2 years of sequential therapy with a bisphosphonate to prevent rapid bone loss.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
If a new fracture occurs when the patients has been treated with denosumab ≥12 months, sequential therapy may include oral or intravenous bisphosphonates or romosozumab.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Romosozumab
Romosozumab is approved for the treatment of osteoporosis in postmenopausal women at high risk for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy, but has not been assessed for the treatment of corticosteroid-induced osteoporosis.[203]Taylor AD, Saag KG. Anabolics in the management of glucocorticoid-induced osteoporosis: an evidence-based review of long-term safety, efficacy and place in therapy. Core Evid. 2019 Aug 23;14:41-50.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6711555
http://www.ncbi.nlm.nih.gov/pubmed/31692480?tool=bestpractice.com
However, the ACR conditionally recommends romosozumab as a treatment for adults aged ≥40 years at moderate to high risk of fracture, including those taking a high dose of corticosteroids (initial prednisolone dose ≥30 mg/day or cumulative prednisolone dose ≥5 g in 1 year) only if they are intolerant of all other treatments.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Romosozumab is not recommended for patients aged <40 years with a moderate to high risk of fracture, and should only be considered in those taking high-dose corticosteroids if other treatments are not tolerated.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Patients treated with romosozumab who are at a low or moderate risk of fracture when romosozumab and corticosteroid treatment is stopped will need sequential therapy with oral or intravenous bisphosphonate, for those who experience a new fracture after ≥12 months of romosozumab treatment, oral or intravenous bisphosphonate or denosumab can be used for sequential therapy.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Patients treated with denosumab for sequential therapy will require a further 6-7 months of bisphosphonate therapy to prevent rapid bone loss and the development of new vertebral compression fractures.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Raloxifene
Evidence on the effectiveness of raloxifene to treat patients with corticosteroid induced osteoporosis is lacking.[204]Popp AW, Isenegger J, Buergi EM, et al. Glucocorticosteroid-induced spinal osteoporosis: scientific update on pathophysiology and treatment. Eur Spine J. 2006 Jul;15(7):1035-49.
http://www.ncbi.nlm.nih.gov/pubmed/16474946?tool=bestpractice.com
However, the ACR conditionally recommends raloxifene as a treatment for adults aged ≥40 years at moderate to high risk of fracture, including those taking a high dose of corticosteroids (initial prednisolone dose ≥30 mg/day or cumulative prednisolone dose ≥5 g in 1 year) only if they are intolerant of all other treatments.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Raloxifene is not recommended for patients aged <40 years with a moderate to high risk of fracture, and should only be considered in those taking high-dose corticosteroids if other treatments are not tolerated.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
Patients treated with raloxifene who are at a low or moderate risk of fracture when raloxifene and corticosteroid treatment is stopped will not require sequential therapy.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com
If a new fracture occurs after treatment with raloxifene for ≥12 months, sequential therapy with oral or intravenous bisphosphonates is needed.[87]Humphrey MB, Russell L, Danila MI, et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023 Dec;75(12):2088-102.
https://acrjournals.onlinelibrary.wiley.com/doi/10.1002/art.42646
http://www.ncbi.nlm.nih.gov/pubmed/37845798?tool=bestpractice.com


 ]
[Evidence A]
]
[Evidence A]