Metabolic dysfunction-associated steatotic liver disease (MASLD) is a spectrum of disease, ranging from hepatic fat accumulation (steatosis) without inflammation, to steatohepatitis, fibrosis, cirrhosis, and end-stage liver disease. Laboratory tests and imaging support the diagnosis. Liver biopsy and histology is the gold standard for diagnosis but is used sparingly because of the associated morbidity. Risk stratification tools are used to identify the patients who are most likely to benefit from liver biopsy. Non-invasive blood-based or imaging-based biomarkers can be used in community settings for risk stratification in the diagnostic evaluation of patients with MASLD.[33]Wattacheril JJ, Abdelmalek MF, Lim JK, et al. AGA clinical practice update on the role of noninvasive biomarkers in the evaluation and management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2023 Oct;165(4):1080-8.
https://www.doi.org/10.1053/j.gastro.2023.06.013
http://www.ncbi.nlm.nih.gov/pubmed/37542503?tool=bestpractice.com
[34]Sterling RK, Duarte-Rojo A, Patel K, et al. AASLD practice guideline on imaging-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_imaging_based.807.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489518?tool=bestpractice.com
[35]Sterling RK, Patel K, Duarte-Rojo A, et al. AASLD practice guideline on blood-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_blood_based.810.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489523?tool=bestpractice.com
[36]Kaplan DE, Ripoll C, Thiele M, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024 May 1;79(5):1180-211.
https://www.doi.org/10.1097/HEP.0000000000000647
http://www.ncbi.nlm.nih.gov/pubmed/37870298?tool=bestpractice.com
A combination of imaging-based and blood-based techniques may be used to detect significant fibrosis and advanced fibrosis, particularly in those undergoing initial fibrosis staging.[34]Sterling RK, Duarte-Rojo A, Patel K, et al. AASLD practice guideline on imaging-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_imaging_based.807.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489518?tool=bestpractice.com
Clinical picture
Patients may report fatigue and malaise or abdominal pain; however, many are asymptomatic.[37]Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346(16):1221-31.
http://www.ncbi.nlm.nih.gov/pubmed/11961152?tool=bestpractice.com
[38]Sharma P, Arora A. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: spectrum and diagnosis. Transl Gastroenterol Hepatol. 2020;5:19.
https://www.doi.org/10.21037/tgh.2019.10.02
http://www.ncbi.nlm.nih.gov/pubmed/32258523?tool=bestpractice.com
The most common presentation is mild abnormality in liver function tests (LFTs) with elevation of alkaline phosphatase, aminotransferases, or bilirubin during a work-up for hypertension, diabetes, or obesity. These may also be found as part of routine blood work for yearly health physicals, or for monitoring medications, most notably anti-hyperlipidaemic therapy. Patients may also present with incidentally detected SLD on abdominal imaging.
In the earlier stages, mild hepatomegaly may be the only abnormality on examination.[39]Bacon BR, Faravash MJ, Janney CG, et al. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994 Oct;107(4):1103-9.
http://www.ncbi.nlm.nih.gov/pubmed/7523217?tool=bestpractice.com
In the end stages of the disease, patients may have characteristic symptoms and signs of chronic liver disease (e.g., jaundice, splenomegaly, ascites, hepatic encephalopathy, oedema, and easy bruising).
The diagnosis of MASLD requires exclusion of alcohol use as the cause of liver disease. Varying levels of significant alcohol intake have been suggested.[40]Liangpunsakul S, Chalasani N. What should we recommend to our patients with NAFLD regarding alcohol use? Am J Gastroenterol. 2012 Jul;107(7):976-8.
https://www.doi.org/10.1038/ajg.2012.20
http://www.ncbi.nlm.nih.gov/pubmed/22764020?tool=bestpractice.com
A standard drink contains 14 g of pure alcohol. The American Association for the Study of Liver Diseases defines mild alcohol intake as up to 20 g/day in women and up to 30 g/day in men, moderate intake as 21-39 g/day in women and 31-59 g/day in men, and heavy intake as ≥40 g/day in women and ≥60 g/day in men.[3]Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
https://journals.lww.com/hep/fulltext/2023/05000/aasld_practice_guidance_on_the_clinical_assessment.31.aspx
http://www.ncbi.nlm.nih.gov/pubmed/36727674?tool=bestpractice.com
Asia-Pacific guidelines define significant alcohol consumption as >140 g/week in men and >70 g/week in women.[8]Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017 - part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018 Jan;33(1):70-85.
https://www.doi.org/10.1111/jgh.13857
http://www.ncbi.nlm.nih.gov/pubmed/28670712?tool=bestpractice.com
European guidelines define significant alcohol consumption as ≥30 g/day in men and ≥20 g/day in women.[41]European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016 Jun;64(6):1388-402.
https://www.doi.org/10.1016/j.jhep.2015.11.004
http://www.ncbi.nlm.nih.gov/pubmed/27062661?tool=bestpractice.com
Alcohol intake should be verified by more than one healthcare worker and on separate occasions to ensure consistency.
A new term 'MetALD' has been coined by the AASLD to describe those with MASLD who consume greater amounts of alcohol per week (140-350 g/week for females and 210-420 g/week for males) and have SLD and cardiometabolic risk factors.[1]Kanwal F, Neuschwander-Tetri BA, Loomba R, et al. Metabolic dysfunction-associated steatotic liver disease: update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology. 2024 May 1;79(5):1212-9.
https://www.doi.org/10.1097/HEP.0000000000000670
http://www.ncbi.nlm.nih.gov/pubmed/38445559?tool=bestpractice.com
[2]American Association for the Study of Liver Diseases. New MASLD nomenclature. Jun 2023 [internet publication].
https://www.aasld.org/new-masld-nomenclature
Medications associated with the development of SLD include oestrogens (tamoxifen), corticosteroids, diltiazem, nifedipine, methotrexate, valproate, griseofulvin, intravenous tetracycline, amiodarone, and antiretroviral therapy for HIV.
Examples of drugs associated with a pure microvesicular histologic phenotype are aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, valproate, tetracycline, nucleoside reverse-transcriptase inhibitors, and cocaine. Drugs associated with a macrovesicular histological phenotype include NSAIDs, corticosteroids, methotrexate, metoprolol, fluorouracil, cisplatin, irinotecan, tamoxifen, and chlorinated hydrocarbons. A mixed macrovesicular and microvesicular histological phenotype may be associated with drugs such as amiodarone, valproate, and methotrexate. Drugs including amiodarone, methotrexate, fluorouracil, cisplatin, irinotecan, and tamoxifen may also be associated with a steatohepatitic histological phenotype.[31]Fontana RJ, Liou I, Reuben A, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2022 Jul 27 [Epub ahead of print].
https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.32689
http://www.ncbi.nlm.nih.gov/pubmed/35899384?tool=bestpractice.com
Long-term methotrexate treatment, in particular, is associated with the development of SLD and fibrosis, particularly in the presence of other known risk factors (obesity, alcohol consumption, pre-existing liver disease, diabetes, hyperlipidaemia). If long-term methotrexate treatment is required, recommendations include restricting its use in patients with suspected MASLD to those who have normal liver biochemistry and do not have advanced fibrosis, laboratory testing at baseline and during treatment, with liver biopsy generally used if transient elastography results are abnormal or abnormal liver biochemistry findings persist.[31]Fontana RJ, Liou I, Reuben A, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2022 Jul 27 [Epub ahead of print].
https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.32689
http://www.ncbi.nlm.nih.gov/pubmed/35899384?tool=bestpractice.com
Obesity, insulin resistance, diabetes, metabolic syndrome, and dyslipidaemia are all associated with MASLD.[7]Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64(1):73-84.
https://www.doi.org/10.1002/hep.28431
http://www.ncbi.nlm.nih.gov/pubmed/26707365?tool=bestpractice.com
Notably, there is a high prevalence of lean MASLD in Asian countries, where up to 19% of patients with MASLD are not obese.[3]Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
https://journals.lww.com/hep/fulltext/2023/05000/aasld_practice_guidance_on_the_clinical_assessment.31.aspx
http://www.ncbi.nlm.nih.gov/pubmed/36727674?tool=bestpractice.com
Lean MASLD should be diagnosed in individuals with MASLD and body mass index <25 kg/m² (non-Asian race) or body mass index <23 kg/m² (Asian race).[42]Long MT, Noureddin M, Lim JK. AGA Clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology. 2022 Sep;163(3):764-74.e1
https://www.gastrojournal.org/article/S0016-5085(22)00628-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35842345?tool=bestpractice.com
In such patients, insulin resistance and the presence of central adiposity may serve as stronger predictors of disease.[43]Liu CJ. Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol. 2012 Oct;27(10):1555-60.
https://onlinelibrary.wiley.com/doi/10.1111/j.1440-1746.2012.07222.x
http://www.ncbi.nlm.nih.gov/pubmed/22741595?tool=bestpractice.com
Laboratory tests
Liver enzymes should be requested for all patients with suspected MASLD.[8]Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017 - part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018 Jan;33(1):70-85.
https://www.doi.org/10.1111/jgh.13857
http://www.ncbi.nlm.nih.gov/pubmed/28670712?tool=bestpractice.com
Elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, between 1 and 4 times the upper limit of normal (ULN) values, occur in 50% to 90% of patients with MASLD.[44]Diehl AM, Goodman Z, Ishak KG. Alcohol-like liver disease in nonalcoholics: a clinical and histological comparison with alcohol-induced liver injury. Gastroenterology. 1988 Oct;95(4):1056-62.
http://www.ncbi.nlm.nih.gov/pubmed/3410220?tool=bestpractice.com
Results rarely exceed 300 IU/L.[45]Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017 Jan;112(1):18-35.
https://www.doi.org/10.1038/ajg.2016.517
http://www.ncbi.nlm.nih.gov/pubmed/27995906?tool=bestpractice.com
Patients with any type of MASLD may have normal LFTs.[38]Sharma P, Arora A. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: spectrum and diagnosis. Transl Gastroenterol Hepatol. 2020;5:19.
https://www.doi.org/10.21037/tgh.2019.10.02
http://www.ncbi.nlm.nih.gov/pubmed/32258523?tool=bestpractice.com
[46]Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003 Jun;37(6):1286-92.
https://www.doi.org/10.1053/jhep.2003.50229
http://www.ncbi.nlm.nih.gov/pubmed/12774006?tool=bestpractice.com
The AST:ALT ratio (AAR) in metabolic dysfunction-associated steatohepatitis (MASH) is typically <1.[47]Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002 Feb;(suppl 17):S186-90.
http://www.ncbi.nlm.nih.gov/pubmed/12000605?tool=bestpractice.com
This differs from acute alcohol-related hepatitis, where the ratio is usually >2. Ratio reversal in patients with MASH (AAR >1) may be an indicator of more advanced liver fibrosis.[37]Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002 Apr 18;346(16):1221-31.
http://www.ncbi.nlm.nih.gov/pubmed/11961152?tool=bestpractice.com
[48]Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999 Dec;30(6):1356-62.
http://www.ncbi.nlm.nih.gov/pubmed/10573511?tool=bestpractice.com
Mild elevations may be seen in alkaline phosphatase and/or gamma glutamyl transferase. Bilirubin is usually normal unless the patient has decompensated chronic liver disease.
Blood should also be sent for full blood count, electrolytes (checking for hyponatraemia), renal function (creatinine and urea), glucose, lipid panel, and coagulation profile.
Fasting insulin (microunits/mL) and the homeostatic model assessment (HOMA) calculation (glucose [mg/dL] × insulin/405) are used to quantify insulin resistance and beta-cell function.
Laboratory tests should be requested to rule out other causes of chronic liver disease. These include:[45]Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017 Jan;112(1):18-35.
https://www.doi.org/10.1038/ajg.2016.517
http://www.ncbi.nlm.nih.gov/pubmed/27995906?tool=bestpractice.com
[49]Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009 Jan;49(1):306-17.
https://www.doi.org/10.1002/hep.22603
http://www.ncbi.nlm.nih.gov/pubmed/19065650?tool=bestpractice.com
Hepatitis C virus antibody
Hepatitis B surface antigen, surface antibody, and core antibody
Iron studies, including iron, total iron-binding capacity, percentage saturation, and ferritin
Autoimmune markers (including antinuclear antibody, smooth muscle antibody, anti-liver kidney microsomal antibody, quantitative immunoglobulins)
Alpha-1 antitrypsin level and phenotype
Anti-M2 mitochondrial antibody to test for primary biliary cholangitis; and ceruloplasmin to screen for Wilson's disease in appropriate age groups (<40 years)
HFE gene mutation testing, if ferritin is elevated, to screen for hereditary haemochromatosis.
In addition, several biochemical definitions of clinically significant drug-induced liver injury have been described and these can help to rule out drug-induced liver injury as a cause of SLD:[31]Fontana RJ, Liou I, Reuben A, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2022 Jul 27 [Epub ahead of print].
https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.32689
http://www.ncbi.nlm.nih.gov/pubmed/35899384?tool=bestpractice.com
Serum AST or ALT >5 × ULN, or alkaline phosphatase (ALP) >2 × ULN (if the baseline is abnormal, use the pretreatment baseline)
Total serum bilirubin >2.5 mg/dL plus an elevated AST, ALT, or ALP level
International normalised ratio (INR) >1.5 with an elevated level of AST, ALT, or ALP.
Fibrosis risk scoring
Several scoring systems have been constructed and validated to help determine which patients with MASLD have the highest risk of progressing to end-stage liver disease, and to help guide decision-making regarding liver biopsy. The NAFLD Fibrosis Score and the Fibrosis 4 Score (FIB-4) are the preferred non-invasive scoring systems for predicting advanced fibrosis.[50]Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a non-invasive system that indentifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854.
http://onlinelibrary.wiley.com/doi/10.1002/hep.21496/full
http://www.ncbi.nlm.nih.gov/pubmed/17393509?tool=bestpractice.com
[51]McPherson S, Armstrong MJ, Cobbold JF, et al. Quality standards for the management of non-alcoholic fatty liver disease (NAFLD): consensus recommendations from the British Association for the Study of the Liver and British Society of Gastroenterology NAFLD Special Interest Group. Lancet Gastroenterol Hepatol. 2022 Aug;7(8):755-69.
https://www.thelancet.com/journals/langas/article/PIIS2468-1253(22)00061-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35490698?tool=bestpractice.com
In adult patients with MASLD who require fibrosis staging, the AASLD recommends using blood-based tests non-invasive over no tests to detect advanced fibrosis.[35]Sterling RK, Patel K, Duarte-Rojo A, et al. AASLD practice guideline on blood-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_blood_based.810.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489523?tool=bestpractice.com
The AASLD suggests against the use of blood-based non-invasive tests to detect steatosis in patients with MASLD.[35]Sterling RK, Patel K, Duarte-Rojo A, et al. AASLD practice guideline on blood-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_blood_based.810.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489523?tool=bestpractice.com
The NAFLD Fibrosis Score utilises age, hyperglycaemia, body mass index (BMI), platelet count, albumin, and AAR to predict patients with advanced liver fibrosis.[50]Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a non-invasive system that indentifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854.
http://onlinelibrary.wiley.com/doi/10.1002/hep.21496/full
http://www.ncbi.nlm.nih.gov/pubmed/17393509?tool=bestpractice.com
NAFLD Fibrosis Score
Opens in new window By applying this model, a liver biopsy may be avoided in a substantial proportion of patients. The FIB-4 index utilises platelet count, age, AST, and ALT.[52]Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009 Oct;7(10):1104-12.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3079239
http://www.ncbi.nlm.nih.gov/pubmed/19523535?tool=bestpractice.com
Fibrosis 4 Score (FIB-4)
Opens in new window Patients with MASLD with a FIB-4 score of <1.3 are unlikely to have advanced fibrosis.[33]Wattacheril JJ, Abdelmalek MF, Lim JK, et al. AGA clinical practice update on the role of noninvasive biomarkers in the evaluation and management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2023 Oct;165(4):1080-8.
https://www.doi.org/10.1053/j.gastro.2023.06.013
http://www.ncbi.nlm.nih.gov/pubmed/37542503?tool=bestpractice.com
The British Association for the Study of the Liver and British Society of Gastroenterology NAFLD Special Interest Group recommends patients at low risk of significant fibrosis should be reassessed every 3 years using non-invasive tests.[51]McPherson S, Armstrong MJ, Cobbold JF, et al. Quality standards for the management of non-alcoholic fatty liver disease (NAFLD): consensus recommendations from the British Association for the Study of the Liver and British Society of Gastroenterology NAFLD Special Interest Group. Lancet Gastroenterol Hepatol. 2022 Aug;7(8):755-69.
https://www.thelancet.com/journals/langas/article/PIIS2468-1253(22)00061-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35490698?tool=bestpractice.com
In patients with MASLD with a FIB-4 score of >1.3, a combination of two or more non-invasive tests (blood-based and/or imaging-based) is preferred for staging and risk stratification.[33]Wattacheril JJ, Abdelmalek MF, Lim JK, et al. AGA clinical practice update on the role of noninvasive biomarkers in the evaluation and management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2023 Oct;165(4):1080-8.
https://www.doi.org/10.1053/j.gastro.2023.06.013
http://www.ncbi.nlm.nih.gov/pubmed/37542503?tool=bestpractice.com
The Enhanced Liver Fibrosis (ELF) score uses a panel of three biomarkers: tissue inhibitor of metalloproteinases 1 (TIMP-1), amino-terminal propeptide of type III procollagen (PIIINP), and hyaluronic acid. The score correlates well with stages of fibrosis in chronic liver disease.[53]Lichtinghagen R, Pietsch D, Bantel H, et al. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013 Aug;59(2):236-42.
https://www.doi.org/10.1016/j.jhep.2013.03.016
http://www.ncbi.nlm.nih.gov/pubmed/23523583?tool=bestpractice.com
Imaging
The American Association for the Study of Liver Diseases (AASLD) recommends using imaging-based non-invasive testing to detect significant fibrosis, advanced fibrosis, and cirrhosis in adults with MASLD.[34]Sterling RK, Duarte-Rojo A, Patel K, et al. AASLD practice guideline on imaging-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_imaging_based.807.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489518?tool=bestpractice.com
Imaging-based tests may be preferentially incorporated into the initial fibrosis staging process owing to their higher accuracy over blood-based techniques and are recommended for the identification of advanced fibrosis and cirrhosis in adults with MASLD.[34]Sterling RK, Duarte-Rojo A, Patel K, et al. AASLD practice guideline on imaging-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_imaging_based.807.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489518?tool=bestpractice.com
Either ultrasound-based transient elastography or magnetic resonance elastography is recommended by the AASLD to stage fibrosis in adults with chronic liver disease.[34]Sterling RK, Duarte-Rojo A, Patel K, et al. AASLD practice guideline on imaging-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_imaging_based.807.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489518?tool=bestpractice.com
The AASLD advises against using imaging-based tests as a standalone test to assess regression or progression of liver fibrosis.[34]Sterling RK, Duarte-Rojo A, Patel K, et al. AASLD practice guideline on imaging-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_imaging_based.807.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489518?tool=bestpractice.com
Imaging studies can determine the presence and amount of fatty infiltration. Ultrasound is a reasonable starting imaging modality.[8]Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017 - part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018 Jan;33(1):70-85.
https://www.doi.org/10.1111/jgh.13857
http://www.ncbi.nlm.nih.gov/pubmed/28670712?tool=bestpractice.com
[41]European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016 Jun;64(6):1388-402.
https://www.doi.org/10.1016/j.jhep.2015.11.004
http://www.ncbi.nlm.nih.gov/pubmed/27062661?tool=bestpractice.com
[54]European Association for the Study of the Liver. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021 Sep;75(3):659-89.
https://www.doi.org/10.1016/j.jhep.2021.05.025
http://www.ncbi.nlm.nih.gov/pubmed/34166721?tool=bestpractice.com
It has a sensitivity of 84.8% and specificity of 93.6% for detecting moderate to severe fatty liver, compared with histology. Controlled attenuation parameter, an ultrasound-based technique, provides a point-of-care semi-quantitative assessment of SLD.[3]Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
https://journals.lww.com/hep/fulltext/2023/05000/aasld_practice_guidance_on_the_clinical_assessment.31.aspx
http://www.ncbi.nlm.nih.gov/pubmed/36727674?tool=bestpractice.com
Transient elastography measured controlled attenuation parameter (TE-CAP) has good diagnostic accuracy to grade steatosis and can be used in clinical practice.[34]Sterling RK, Duarte-Rojo A, Patel K, et al. AASLD practice guideline on imaging-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_imaging_based.807.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489518?tool=bestpractice.com
Magnetic resonance imaging (MRI) liver may be requested if SLD is suspected but is not detected using ultrasound.[8]Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017 - part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018 Jan;33(1):70-85.
https://www.doi.org/10.1111/jgh.13857
http://www.ncbi.nlm.nih.gov/pubmed/28670712?tool=bestpractice.com
MRI protein density fat fraction (MRI-PDFF) correlates well with biopsy-proven SLD and is more accurate than ultrasound for identifying SLD.[55]Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013 Jun;267(3):767-75.
https://www.doi.org/10.1148/radiol.13121360
http://www.ncbi.nlm.nih.gov/pubmed/23382293?tool=bestpractice.com
[56]Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017 Feb;152(3):598-607.e2.
http://www.ncbi.nlm.nih.gov/pubmed/27911262?tool=bestpractice.com
MRI-PDFF can quantify steatosis.[3]Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
https://journals.lww.com/hep/fulltext/2023/05000/aasld_practice_guidance_on_the_clinical_assessment.31.aspx
http://www.ncbi.nlm.nih.gov/pubmed/36727674?tool=bestpractice.com
However, availability is limited.
Imaging-based non-invasive tests, such as TE-CAP and MRI-PDFF or magnetic resonance spectroscopy, are superior to blood-based non-invasive tests and should be used in the assessment of hepatic steatosis in adults with MASLD, where available.[34]Sterling RK, Duarte-Rojo A, Patel K, et al. AASLD practice guideline on imaging-based non-invasive liver disease assessments of hepatic fibrosis and steatosis. Hepatology. 15 Mar 2024 [Epub ahead of print].
https://journals.lww.com/hep/citation/9900/aasld_practice_guideline_on_imaging_based.807.aspx
http://www.ncbi.nlm.nih.gov/pubmed/38489518?tool=bestpractice.com
For the identification of advanced fibrosis or cirrhosis, the leading biomarker is liver stiffness. Ultrasound shear wave elastography is a non-invasive technique for assessing liver stiffness. Tissue stiffness is deduced from analysis of shear waves that are generated by high-intensity ultrasound pulses.[57]American College of Radiology. ACR appropriateness criteria: chronic liver disease. 2019 [internet publication].
https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria
Magnetic resonance elastography is an alternative technique for assessing liver stiffness. It is more accurate than ultrasound shear wave elastography for identifying fibrosis and cirrhosis, and performs better in people with obesity.[54]European Association for the Study of the Liver. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021 Sep;75(3):659-89.
https://www.doi.org/10.1016/j.jhep.2021.05.025
http://www.ncbi.nlm.nih.gov/pubmed/34166721?tool=bestpractice.com
[56]Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017 Feb;152(3):598-607.e2.
http://www.ncbi.nlm.nih.gov/pubmed/27911262?tool=bestpractice.com
[57]American College of Radiology. ACR appropriateness criteria: chronic liver disease. 2019 [internet publication].
https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria
Liver biopsy
Liver biopsy is the gold standard for confirming the diagnosis of MASLD. It is also the most sensitive and specific means of providing important prognostic information.[Figure caption and citation for the preceding image starts]: A wedge biopsy of the liver from a 52-year-old female organ donor; the biopsy shows moderate mixed micro- and macrovesicular steatosis; there is no significant lobular inflammation or necrosis (haematoxylin and eosin, [H&E] stain, x 200)From the collection of Kapil B. Chopra, MD [Citation ends].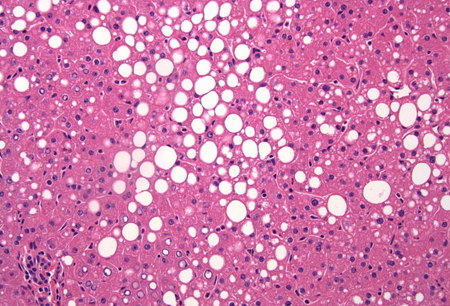 [Figure caption and citation for the preceding image starts]: A case of metabolic dysfunction-associated steatohepatitis; the biopsy shows ballooning degeneration of the hepatocytes (middle right) and spotty lobular inflammation in addition to mixed micro- and macrovesicular steatosis (H&E, x 200)From the collection of Kapil B. Chopra, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: A case of metabolic dysfunction-associated steatohepatitis; the biopsy shows ballooning degeneration of the hepatocytes (middle right) and spotty lobular inflammation in addition to mixed micro- and macrovesicular steatosis (H&E, x 200)From the collection of Kapil B. Chopra, MD [Citation ends].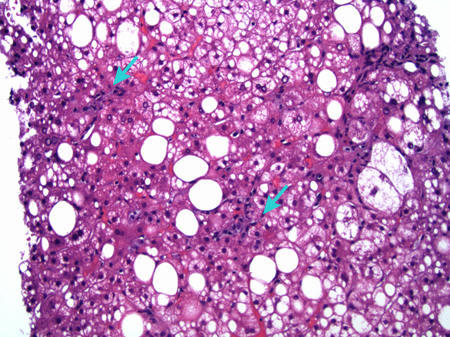 When disease is likely to be benign (normal liver function tests with imaging showing mild fatty infiltration), a biopsy is probably not necessary. Staging for MASLD can now be performed by a combination of radiological and laboratory techniques, greatly reducing the requirement for invasive liver biopsy in most patients.[58]Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011 Mar;33(5):525-40.
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2036.2010.04556.x/full
http://www.ncbi.nlm.nih.gov/pubmed/21198708?tool=bestpractice.com
Liver biopsy is usually reserved for selected patients, including:[3]Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
https://journals.lww.com/hep/fulltext/2023/05000/aasld_practice_guidance_on_the_clinical_assessment.31.aspx
http://www.ncbi.nlm.nih.gov/pubmed/36727674?tool=bestpractice.com
[8]Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017 - part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018 Jan;33(1):70-85.
https://www.doi.org/10.1111/jgh.13857
http://www.ncbi.nlm.nih.gov/pubmed/28670712?tool=bestpractice.com
When disease is likely to be benign (normal liver function tests with imaging showing mild fatty infiltration), a biopsy is probably not necessary. Staging for MASLD can now be performed by a combination of radiological and laboratory techniques, greatly reducing the requirement for invasive liver biopsy in most patients.[58]Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011 Mar;33(5):525-40.
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2036.2010.04556.x/full
http://www.ncbi.nlm.nih.gov/pubmed/21198708?tool=bestpractice.com
Liver biopsy is usually reserved for selected patients, including:[3]Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023 May 1;77(5):1797-835.
https://journals.lww.com/hep/fulltext/2023/05000/aasld_practice_guidance_on_the_clinical_assessment.31.aspx
http://www.ncbi.nlm.nih.gov/pubmed/36727674?tool=bestpractice.com
[8]Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017 - part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018 Jan;33(1):70-85.
https://www.doi.org/10.1111/jgh.13857
http://www.ncbi.nlm.nih.gov/pubmed/28670712?tool=bestpractice.com
Patients with MASLD who are at increased risk of having steatohepatitis and/or advanced fibrosis (e.g., patients with metabolic syndrome, patients with elevated NAFLD Fibrosis Score or FIB-4 index, or increased liver stiffness on imaging)
Patients with a possible alternative cause of SLD or coexisting chronic liver disease (if these alternative causes/coexisting diagnoses cannot be excluded by other means). In patients with suspected MASLD and antinuclear antibody positivity at titres greater than 1:160 or anti-smooth muscle antibody positivity at titres greater than 1:40, a liver biopsy may be considered to exclude the presence of autoimmune hepatitis[49]Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009 Jan;49(1):306-17.
https://www.doi.org/10.1002/hep.22603
http://www.ncbi.nlm.nih.gov/pubmed/19065650?tool=bestpractice.com
Patients in whom non-invasive assessment of liver fibrosis is inconclusive
Patients who have persistent elevations (>6 months) in liver enzymes despite lifestyle changes
Patients who are being considered for entry into a clinical trial.
The decision to perform a liver biopsy in a patient with suspected MASLD, as well as the timing of the biopsy, should be an individualised, shared decision between patient and hepatologist. The American Gastroenterological Association advises liver biopsy in patients with non-invasive test results that are indeterminate or discordant or conflict with other clinical, laboratory, or radiological findings or when alternative aetiologies for liver disease are suspected.[33]Wattacheril JJ, Abdelmalek MF, Lim JK, et al. AGA clinical practice update on the role of noninvasive biomarkers in the evaluation and management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2023 Oct;165(4):1080-8.
https://www.doi.org/10.1053/j.gastro.2023.06.013
http://www.ncbi.nlm.nih.gov/pubmed/37542503?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: A case of metabolic dysfunction-associated steatohepatitis; the biopsy shows ballooning degeneration of the hepatocytes (middle right) and spotty lobular inflammation in addition to mixed micro- and macrovesicular steatosis (H&E, x 200)From the collection of Kapil B. Chopra, MD [Citation ends].
[Figure caption and citation for the preceding image starts]: A case of metabolic dysfunction-associated steatohepatitis; the biopsy shows ballooning degeneration of the hepatocytes (middle right) and spotty lobular inflammation in addition to mixed micro- and macrovesicular steatosis (H&E, x 200)From the collection of Kapil B. Chopra, MD [Citation ends]. When disease is likely to be benign (normal liver function tests with imaging showing mild fatty infiltration), a biopsy is probably not necessary. Staging for MASLD can now be performed by a combination of radiological and laboratory techniques, greatly reducing the requirement for invasive liver biopsy in most patients.[58] Liver biopsy is usually reserved for selected patients, including:[3][8]
When disease is likely to be benign (normal liver function tests with imaging showing mild fatty infiltration), a biopsy is probably not necessary. Staging for MASLD can now be performed by a combination of radiological and laboratory techniques, greatly reducing the requirement for invasive liver biopsy in most patients.[58] Liver biopsy is usually reserved for selected patients, including:[3][8]