Summary
Definition
History and exam
Key diagnostic factors
- presence of risk factors
- malaise
- fever
- diarrhoea
- nausea and vomiting
- visual floaters and blindness
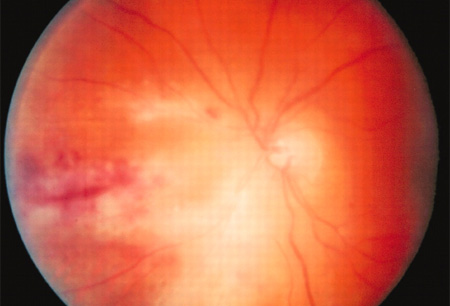
- abnormalities on fundoscopy
- newborn: microcephaly
- newborn: poor tone and motor function and abnormal head lag
- newborn: hearing loss
Other diagnostic factors
- newborn: hepatosplenomegaly
- newborn: petechiae or purpura
- pain and weakness
Risk factors
- CMV D+/R- status (donor CMV seropositive, recipient seronegative) in solid organ transplant recipients
- CMV R+ (recipient seropositive) in transplant recipients
- type of immunosuppressive drugs
- AIDS
- inflammatory bowel disease
- acute illness in intensive care setting
- newborn in CMV infection during pregnancy
Diagnostic investigations
1st investigations to order
- FBC
- serum creatinine
- serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
- serum alkaline phosphatase
- serology
- pp65 antigenaemia
- nucleic acid detection
- CD4 count
- chest x-ray
Investigations to consider
- chest CT scan
- histopathology of biopsy
- upper gastrointestinal endoscopy and colonoscopy
- serial fetal ultrasound examinations (congenital CMV infection)
- amniocentesis or fetal blood sampling (congenital CMV infection)
- brain ultrasound/brain MRI: newborns
Treatment algorithm
Contributors
Authors
Matteo Mombelli, MD
Infectious Disease Service and Transplantation Center
University Hospital (CHUV)
University of Lausanne
Lausanne
Switzerland
Disclosures
MM declares that he has no competing interests.
Oriol Manuel, MD
Infectious Disease Service and Transplantation Center
University Hospital (CHUV)
University of Lausanne
Lausanne
Switzerland
Disclosures
OM has participated in Advisory Boards of MSD, Takeda, Astra-Zeneca, and Biotest.
Acknowledgements
Dr Matteo Mombelli and Dr Oriol Manuel would like to thank Dr Sandra Asner from the University Hospital of Lausanne, Switzerland for her careful review of congenital CMV in this topic. They would also like to gratefully acknowledge Dr Raymund R. Razonable and Dr Atul Humar, the previous contributors to this topic.
Disclosures
RRR is an author of a number of references cited in this topic. AH has done consultancy work for Astellas, Chimerix, and Roche.
Peer reviewers
Joseph Sassine, MD
Assistant Professor of Medicine
Transplant Infectious Diseases
University of Oklahoma Health Sciences Center
Oklahoma City
OK
Disclosures
JS reports research grants paid to his institution from Ansun BioPharma, Cidara Therapeutics, Community Infusion Solutions, F2G, and Shionogi.
Simon Barton, MD, FRCOG, FRCPEd, FRCP
Clinical Director
HIV and Sexual Health
Chelsea and Westminster Hospital
London
UK
Disclosures
SB has been funded by GlaxoSmithKline and Gilead for speaking at scientific meetings.
Use of this content is subject to our disclaimer