Primary adrenal insufficiency (PAI) can be difficult to diagnose. Clinical manifestations are based on the deficiency of adrenocortical hormones (glucocorticoids, mineralocorticoids) and adrenal androgens.[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
It presents with non-specific signs and symptoms, such as fatigue, weakness, and weight loss, that are also common to other diseases. Other manifestations include hyperpigmentation, postural hypotension due to salt loss and dehydration, hyponatraemia, hyperkalaemia, changes in blood count (anaemia, eosinophilia, and lymphocytosis), and hypoglycaemia.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
A high percentage of patients are diagnosed after a life-threatening adrenal crisis.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
Patients presenting with features of adrenal crisis (i.e., hypotension, circulatory failure) should be treated urgently.[26]Arlt W; Society for Endocrinology Clinical Committee. Society For Endocrinology endocrine emergency guidance: emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr Connect. 2016 Sep;5(5):G1-3.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5314805
http://www.ncbi.nlm.nih.gov/pubmed/27935813?tool=bestpractice.com
Tests may be taken as baseline, but diagnostic tests should not delay treatment.[26]Arlt W; Society for Endocrinology Clinical Committee. Society For Endocrinology endocrine emergency guidance: emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr Connect. 2016 Sep;5(5):G1-3.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5314805
http://www.ncbi.nlm.nih.gov/pubmed/27935813?tool=bestpractice.com
Groups of patients with known causative factors for developing PAI should be viewed as being at high risk so that a diagnosis can be established early. Risk factors include female sex and known autoimmune diseases, such as type 1 diabetes, rheumatoid arthritis, pernicious anaemia, coeliac disease, vitiligo, or autoimmune thyroid disease.[3]Husebye ES, Pearce SH, Krone NP, et al. Adrenal insufficiency. Lancet. 2021 Feb 13;397(10274):613-29.
http://www.ncbi.nlm.nih.gov/pubmed/33484633?tool=bestpractice.com
[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
[13]Dalin F, Nordling Eriksson G, Dahlqvist P, et al. Clinical and immunological characteristics of autoimmune Addison disease: a nationwide Swedish multicenter study. J Clin Endocrinol Metab. 2017 Feb 1;102(2):379-89.
https://www.doi.org/10.1210/jc.2016-2522
http://www.ncbi.nlm.nih.gov/pubmed/27870550?tool=bestpractice.com
Another risk factor for the development of PAI is the occurrence of adrenal haemorrhage or haemorrhagic infarction. This can be caused by thromboembolic and/or hypercoagulable states, such as antiphospholipid syndrome, sepsis, and heparin-induced thrombocytopenia.[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
[17]Rosenberger LH, Smith PW, Sawyer RG, et al. Bilateral adrenal hemorrhage: the unrecognized cause of hemodynamic collapse associated with heparin-induced thrombocytopenia. Crit Care Med. 2011 Apr;39(4):833-8.
http://www.ncbi.nlm.nih.gov/pubmed/21242799?tool=bestpractice.com
Patients taking anticoagulants are at increased risk of adrenal haemorrhage.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
Secondary adrenal insufficiency can be associated with severe cortisol deficiency, but otherwise has a different clinical presentation (e.g., no increased pigmentation and only mild mineralocorticoid deficiency). Therefore, the finding of hypocortisolism is not unique to PAI. A history of treatment with glucocorticoid in recent months, or the possibility of hypothalamic-pituitary disease (e.g., concurrent emergence of other secondary pituitary deficiencies, such as thyroid or gonadal deficiencies), should prompt evaluation for secondary adrenal insufficiency.
History
Fatigue and lethargy (or weakness) are the most common presenting symptoms.[3]Husebye ES, Pearce SH, Krone NP, et al. Adrenal insufficiency. Lancet. 2021 Feb 13;397(10274):613-29.
http://www.ncbi.nlm.nih.gov/pubmed/33484633?tool=bestpractice.com
They are usually insidious and may be accompanied by variable degrees of generalised muscle weakness, myalgias, and arthralgias. Patients complain about gradual loss of energy as the day goes on.
Anorexia is often present and may lead to significant weight loss. Both are important features in patients with PAI.
Dizziness, arthralgia, and myalgia are less common.
Gastrointestinal symptoms including nausea, vomiting, and vague abdominal pain are non-specific presenting symptoms, and can suggest adrenal crisis if acutely worsening.[3]Husebye ES, Pearce SH, Krone NP, et al. Adrenal insufficiency. Lancet. 2021 Feb 13;397(10274):613-29.
http://www.ncbi.nlm.nih.gov/pubmed/33484633?tool=bestpractice.com
Salt craving may be present.[3]Husebye ES, Pearce SH, Krone NP, et al. Adrenal insufficiency. Lancet. 2021 Feb 13;397(10274):613-29.
http://www.ncbi.nlm.nih.gov/pubmed/33484633?tool=bestpractice.com
History should be directed at establishing the underlying cause, such as a known history of autoimmune disease, HIV infection, possible tuberculosis infection, and current medication use.
Sudden alterations in glycaemic control and recurrent hypoglycaemia in patients with type 1 diabetes mellitus may suggest autoimmune polyglandular syndrome type 2 with co-existing diabetes and PAI.[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
Physical examination
Most patients present with significant weight loss secondary to anorexia.
The most distinctive feature of PAI is generalised mucosal and cutaneous hyperpigmentation, which is more pronounced in areas of increased friction, such as palms, knuckles, and scars.[3]Husebye ES, Pearce SH, Krone NP, et al. Adrenal insufficiency. Lancet. 2021 Feb 13;397(10274):613-29.
http://www.ncbi.nlm.nih.gov/pubmed/33484633?tool=bestpractice.com
This is due to high levels of adrenocorticotrophic hormone (ACTH), which stimulate dermal melanocortin receptors. [Figure caption and citation for the preceding image starts]: Generally hyperpigmented young man with primary adrenal insufficiency: exaggerated pigmentation over pressure points on elbowsFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends].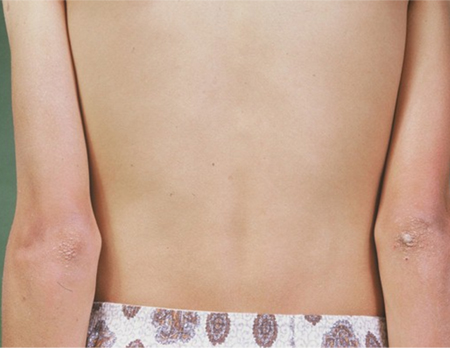 [Figure caption and citation for the preceding image starts]: Hands of patient with primary adrenal insufficiency showing: hyperpigmentation exaggerated on sun-exposed dorsal surface and creases; area of vitiligo in skin over left second metacarpophalangeal jointFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Hands of patient with primary adrenal insufficiency showing: hyperpigmentation exaggerated on sun-exposed dorsal surface and creases; area of vitiligo in skin over left second metacarpophalangeal jointFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends].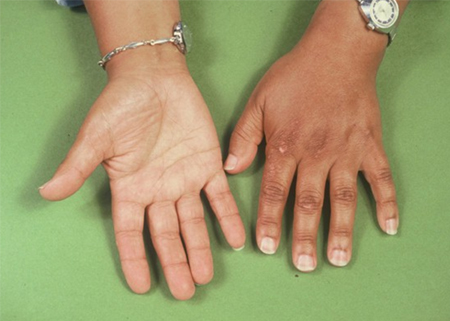 [Figure caption and citation for the preceding image starts]: Area of buccal hyperpigmentation where teeth pinch mucous membrane while chewingFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Area of buccal hyperpigmentation where teeth pinch mucous membrane while chewingFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends].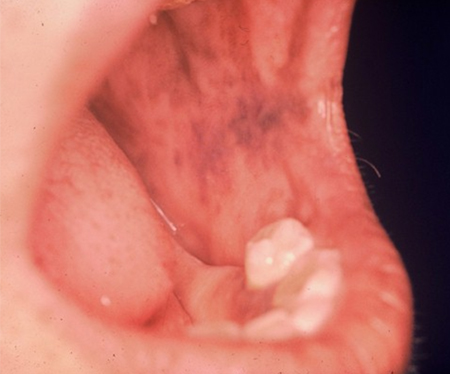
Postural hypotension caused by mineralocorticoid deficiency is common.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
In premenopausal women, loss of adrenal androgens results in significant loss of axillary and pubic hair.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
Other physical signs of autoimmunity, such as vitiligo, premature greying of the hair, and Hashimoto's thyroiditis goitre may be present.
Laboratory evaluation
In an emergency situation, the physician should not wait for the results of blood tests before administering treatment. In acutely ill patients with otherwise unexplained symptoms or signs suggestive of adrenal insufficiency, blood is drawn for baseline ACTH and cortisol level and treatment is administered immediately.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
Further investigations are appropriate when in a less urgent situation to confirm the adrenal insufficiency and determine the aetiology.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
In addition to measurement of serum cortisol level, investigations focus on the integrity of the main peptide stimulating the adrenal cortex (plasma ACTH), as well as the classes of hormones secreted by the adrenal cortex: glucocorticoids (cortisol); mineralocorticoids (aldosterone); and adrenal androgens (dehydroepiandrosterone [DHEA], as well as its sulfated conjugate, DHEA-S).[3]Husebye ES, Pearce SH, Krone NP, et al. Adrenal insufficiency. Lancet. 2021 Feb 13;397(10274):613-29.
http://www.ncbi.nlm.nih.gov/pubmed/33484633?tool=bestpractice.com
It is expected that with PAI there will be loss of cortisol, aldosterone, and DHEA/DHEA-S secretion from the adrenal cortex and that this will be associated with a compensatory rise in plasma ACTH. In contrast, patients with central adrenal insufficiency (that is, a secondary or tertiary cause), will lose ACTH secretion and consequently will have decreased secretion of cortisol as well as DHEA/ DHEA-S while mineralocorticoid function (aldosterone) remains relatively intact.[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
Measurements of cortisol and ACTH levels
Cortisol
The first diagnostic test is measurement of serum cortisol level, preferably in the morning when it is expected to be normally the highest.[27]Karaca Z, Grossman A, Kelestimur F. Investigation of the hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis. Rev Endocr Metab Disord. 2021 Jun;22(2):179-204.
http://www.ncbi.nlm.nih.gov/pubmed/33770352?tool=bestpractice.com
A random serum cortisol level is also acceptable as long as it is interpreted within the context of the patient’s activity and level of stress. This can be used as a test of exclusion.
Cortisol values vary according to the assay used, and use of the reference ranges provided by the relevant laboratory is recommended.[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
Serum cortisol is 80% bound to cortisol-binding globulin (CBG) and 10% to 15% to albumin, so disorders that affect CBG levels or albumin influence cortisol levels.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
CBG levels are affected by oestrogen levels, some medical conditions (e.g., liver disease or nephrotic syndrome), and some heritable mutations.[8]Barthel A, Benker G, Berens K, et al. An update on Addison's disease. Exp Clin Endocrinol Diabetes. 2019 Feb;127(2-03):165-75.
https://www.doi.org/10.1055/a-0804-2715
http://www.ncbi.nlm.nih.gov/pubmed/30562824?tool=bestpractice.com
A level below 140 nanomols/L (5 micrograms/dL) is highly suggestive of adrenal insufficiency.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
A lower cut-off of 85 nanomols/L (3 micrograms/dL) may reduce the number of patients requiring stimulation tests.[27]Karaca Z, Grossman A, Kelestimur F. Investigation of the hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis. Rev Endocr Metab Disord. 2021 Jun;22(2):179-204.
http://www.ncbi.nlm.nih.gov/pubmed/33770352?tool=bestpractice.com
A cortisol level above 500 nanomols/L (18 micrograms/dL) effectively excludes the diagnosis of adrenal insufficiency.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
[28]Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008 Nov;93(11):4245-53.
https://academic.oup.com/jcem/article/93/11/4245/2627237
http://www.ncbi.nlm.nih.gov/pubmed/18697868?tool=bestpractice.com
Patients with suspected adrenal insufficiency and cortisol levels of 140 nanomols/L to 500 nanomols/L (5 to 18 micrograms/dL) require further testing with a high-dose ACTH stimulation test to confirm adrenal functioning.[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
ACTH
Plasma ACTH level should be determined along with a serum cortisol determination.
The presence of a low serum cortisol together with an elevated plasma ACTH (two-times the upper limit of the plasma ACTH reference interval) is indicative of PAI.[3]Husebye ES, Pearce SH, Krone NP, et al. Adrenal insufficiency. Lancet. 2021 Feb 13;397(10274):613-29.
http://www.ncbi.nlm.nih.gov/pubmed/33484633?tool=bestpractice.com
[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
A similar biochemical set of data can at times be observed in patients recovering from chronic suppression of the hypothalamo-pituitary-adrenal (HPA) axis.
Patients with central adrenal insufficiency will have low or low-normal ACTH levels along with low/low normal cortisol values.[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
ACTH stimulation test
The optimal cortisol values need to be individualised depending on the assay used, as newer assays (e.g., monoclonal antibody immunoassays or mass spectrometry) may have a lower threshold and result in a false positive test.[29]Javorsky BR, Raff H, Carroll TB, et al. New cutoffs for the biochemical diagnosis of adrenal insufficiency after ACTH stimulation using specific cortisol assays. J Endocr Soc. 2021 Apr 1;5(4):bvab022.
https://www.doi.org/10.1210/jendso/bvab022
http://www.ncbi.nlm.nih.gov/pubmed/33768189?tool=bestpractice.com
The value of 500 nanomols/L (18 micrograms/dL) is derived from polyclonal antibody assays. Cut-off values also need to be individualised according to the type of ACTH stimulation test used.[30]Cho HY, Kim JH, Kim SW, et al. Different cut-off values of the insulin tolerance test, the high-dose short Synacthen test (250 μg) and the low-dose short Synacthen test (1 μg) in assessing central adrenal insufficiency. Clin Endocrinol (Oxf). 2014 Jul;81(1):77-84.
http://www.ncbi.nlm.nih.gov/pubmed/24382108?tool=bestpractice.com
If cortisol levels below 500 nanomols/L (18 micrograms/dL) are found at either 30 or 60 minutes after ACTH stimulation, the diagnosis of adrenal insufficiency is highly likely.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
Cortisol levels exceeding 500 nanomols/L (18 micrograms/dL) at 30 or 60 minutes after administration of high-dose ACTH (250 micrograms) exclude the diagnosis of PAI in most instances.[8]Barthel A, Benker G, Berens K, et al. An update on Addison's disease. Exp Clin Endocrinol Diabetes. 2019 Feb;127(2-03):165-75.
https://www.doi.org/10.1055/a-0804-2715
http://www.ncbi.nlm.nih.gov/pubmed/30562824?tool=bestpractice.com
However, some patients with mild or early PAI or partial central adrenal insufficiency manifest an adequate increase in cortisol levels after a high-dose ACTH stimulation test.[27]Karaca Z, Grossman A, Kelestimur F. Investigation of the hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis. Rev Endocr Metab Disord. 2021 Jun;22(2):179-204.
http://www.ncbi.nlm.nih.gov/pubmed/33770352?tool=bestpractice.com
[31]Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, et al. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998 Nov;159(2):275-80.
https://joe.bioscientifica.com/view/journals/joe/159/2/275.xml
http://www.ncbi.nlm.nih.gov/pubmed/9795368?tool=bestpractice.com
In situations where this is suspected, patients should have the stimulation test repeated using a low dose of ACTH (1 microgram).[28]Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008 Nov;93(11):4245-53.
https://academic.oup.com/jcem/article/93/11/4245/2627237
http://www.ncbi.nlm.nih.gov/pubmed/18697868?tool=bestpractice.com
A meta-analysis has concluded that the high-dose and low-dose ACTH stimulation tests are satisfactory for indicating the presence of secondary adrenal insufficiency but data in PAI are insufficient to estimate diagnostic accuracy, such that neither was reliable for consistently encoding the disorder.[32]Ospina NS, Al Nofal A, Bancos I, et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016 Feb;101(2):427-34.
https://academic.oup.com/jcem/article/101/2/427/2810551
http://www.ncbi.nlm.nih.gov/pubmed/26649617?tool=bestpractice.com
Patients who have an abnormal ACTH stimulation test consistent with adrenal insufficiency require further laboratory evaluation to determine the type of adrenal insufficiency.
Plasma ACTH level should have been already measured. If high, the cause is PAI. A low or low-normal value would support a diagnosis of secondary adrenal insufficiency.[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
[9]Betterle C, Presotto F, Furmaniak J. Epidemiology, pathogenesis, and diagnosis of Addison's disease in adults. J Endocrinol Invest. 2019 Dec;42(12):1407-33.
http://www.ncbi.nlm.nih.gov/pubmed/31321757?tool=bestpractice.com
Measurements of plasma renin activity and serum aldosterone levels are important when PAI is suspected.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
In patients with PAI the entire adrenal cortex is involved, and biosynthesis of aldosterone is compromised in addition to that of cortisol. To compensate, renin levels become elevated, but they are ineffective. A low plasma aldosterone associated with a high simultaneously measured plasma renin activity is indicative of PAI.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
In contrast, in secondary adrenal insufficiency the renin-angiotensin-aldosterone axis is intact, although slightly less responsive as a consequence of suppressed cortisol concentrations.
Measurement of DHEA/DHEA-S levels is helpful in the assessment of adrenal insufficiency as these adrenal androgens are the first to be lost when adrenal cortical function is impaired.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
[27]Karaca Z, Grossman A, Kelestimur F. Investigation of the hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis. Rev Endocr Metab Disord. 2021 Jun;22(2):179-204.
http://www.ncbi.nlm.nih.gov/pubmed/33770352?tool=bestpractice.com
Levels below the lower limit of normal for age and sex are a useful initial sign of PAI, but cannot be used alone to make the diagnosis because levels may be low, especially in older age, without PAI.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
Since the secretion of DHEA/DHEA-S is ACTH dependent, their levels will also be low in central adrenal insufficiency. Thus, a low DHEA-S level may indicate adrenal failure but will not identify whether it is primary or central in origin. Conversely, a normal serum DHEA-S level makes the diagnosis of adrenal insufficiency (primary or central) very unlikely.[33]Nasrallah MP, Arafah BM. The value of dehydroepiandrosterone sulfate measurements in the assessment of adrenal function. J Clin Endocrinol Metab. 2003 Nov;88(11):5293-8.
https://www.doi.org/10.1210/jc.2003-030449
http://www.ncbi.nlm.nih.gov/pubmed/14602764?tool=bestpractice.com
[34]Sayyed Kassem L, El Sibai K, Chaiban J, et al. Measurements of serum DHEA and DHEA sulphate levels improve the accuracy of the low-dose cosyntropin test in the diagnosis of central adrenal insufficiency. J Clin Endocrinol Metab. 2012 Oct;97(10):3655-62.
https://www.doi.org/10.1210/jc.2012-1806
http://www.ncbi.nlm.nih.gov/pubmed/22851486?tool=bestpractice.com
Ancillary tests
Baseline blood tests (full blood count, electrolytes, urea, creatinine) should be performed, as destruction of the adrenal cortex, and especially deficiency in glucocorticoids and mineralocorticoids, leads to changes in fluid and electrolyte balance and changes in blood count.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
[26]Arlt W; Society for Endocrinology Clinical Committee. Society For Endocrinology endocrine emergency guidance: emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr Connect. 2016 Sep;5(5):G1-3.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5314805
http://www.ncbi.nlm.nih.gov/pubmed/27935813?tool=bestpractice.com
Autoimmune adrenalitis is the most common cause of adult cases of PAI.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
Antibodies directed against the adrenal enzyme 21-hydroxylase are associated with autoimmune PAI, but although sensitive they are not necessary for the diagnosis.[2]Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880116
http://www.ncbi.nlm.nih.gov/pubmed/26760044?tool=bestpractice.com
[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
[35]Betterle C, Dal Pra C, Mantero F, et al. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002 Jun;23(3):327-64.
https://academic.oup.com/edrv/article/23/3/327/2424246
http://www.ncbi.nlm.nih.gov/pubmed/12050123?tool=bestpractice.com
In patients where the underlying cause is still uncertain, imaging of the adrenals using computed tomography and magnetic resonance imaging is recommended to determine other causes, such as infection, haemorrhage, or metastatic disease.[4]Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021 Mar 11;7(1):19.
http://www.ncbi.nlm.nih.gov/pubmed/33707469?tool=bestpractice.com
If secondary adrenal insufficiency is suspected, but the cortisol response to ACTH is not diagnostic, a test of the entire HPA axis may be helpful (e.g., the insulin tolerance test or the overnight metyrapone test). The insulin tolerance test measures the cortisol response to insulin-induced hypoglycaemia.[28]Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008 Nov;93(11):4245-53.
https://academic.oup.com/jcem/article/93/11/4245/2627237
http://www.ncbi.nlm.nih.gov/pubmed/18697868?tool=bestpractice.com
[36]Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998 Jul;83(7):2350-4.
https://academic.oup.com/jcem/article/83/7/2350/2865342
http://www.ncbi.nlm.nih.gov/pubmed/9661607?tool=bestpractice.com
Vigilance is required, due to the fact that hypoglycaemia is an endpoint. The test is contraindicated in older adults and in people with cardiovascular disease or seizure disorder. Another option to investigate the HPA axis is the overnight metyrapone test which measures the 11-deoxycortisol and cortisol response to metyrapone.[37]Fiad TM, Kirby JM, Cunningham SK, et al. The overnight single-dose metyrapone test is a simple and reliable index of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1994 May;40(5):603-9.
http://www.ncbi.nlm.nih.gov/pubmed/8013141?tool=bestpractice.com
Unfortunately, there is limited availability of metyrapone in the US; it can be obtained via a specialist pharmacy. In many countries, metyrapone is available through its manufacturer.
 [Figure caption and citation for the preceding image starts]: Hands of patient with primary adrenal insufficiency showing: hyperpigmentation exaggerated on sun-exposed dorsal surface and creases; area of vitiligo in skin over left second metacarpophalangeal jointFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Hands of patient with primary adrenal insufficiency showing: hyperpigmentation exaggerated on sun-exposed dorsal surface and creases; area of vitiligo in skin over left second metacarpophalangeal jointFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Area of buccal hyperpigmentation where teeth pinch mucous membrane while chewingFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Area of buccal hyperpigmentation where teeth pinch mucous membrane while chewingFrom the personal collection of T. Joseph McKenna, MD; used with permission [Citation ends].