History and exam
Key diagnostic factors
common
presence of risk factors
All of the following are strongly associated with the development of DN: poorly controlled hyperglycaemia, older age (e.g., >70 years), prolonged duration of diabetes (e.g., >10 years), and tall stature.
In addition, dyslipidaemia with elevated triglycerides and hypertension are emerging as strong independent risk factors.[35][36][37]
In people with type 2 diabetes, co-existence of several risk factors for cardiovascular disease and the presence of coronary artery disease are also associated with DN.[54]
pain (peripheral)
Particularly troubling to most patients. Often the reason why patients with DN seek medical care.
May be described as sticking, lancinating, prickling, burning, aching, boring, or excessively sensitive. It is often worse at night and may disturb sleep.
Involvement of larger fibres produces a tight, band-like feeling around the extremity, or an electrical tingling sensation. Involvement of small fibres usually produces burning-like pain.
loss of sensation (peripheral)
Defined in terms of extent, distribution, and modality.
Assessment includes pinprick sensation, light touch, vibration, and joint position.[Figure caption and citation for the preceding image starts]: Light touch testing with monofilamentCreated by the BMJ Group [Citation ends].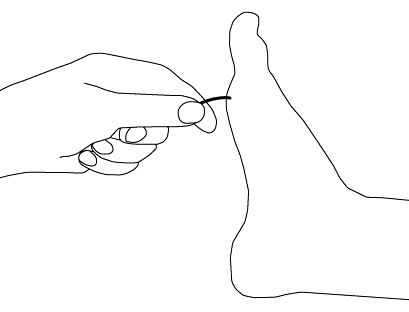 [Figure caption and citation for the preceding image starts]: Vibratory testingCreated by the BMJ Group [Citation ends].
[Figure caption and citation for the preceding image starts]: Vibratory testingCreated by the BMJ Group [Citation ends].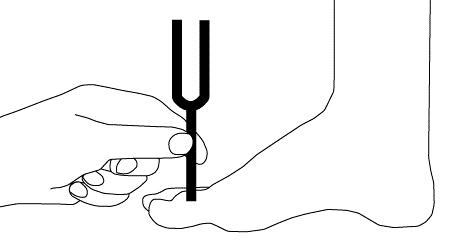
At an early stage sensory loss affects tips of toes and/or fingers. Proceeds proximally, causing a symmetrical distal sensory loss in a 'stocking-glove' pattern.[Figure caption and citation for the preceding image starts]: Progressive axonal loss in diabetic peripheral neuropathyEdwards JL, et al. Pharmacol Ther. 2008 Oct;120(1):1-34; used with permission [Citation ends].
Among patients who complain only of distal burning, the loss of distal sensation may be very subtle. Feeling may also be lost in the feet, with or without pain. Painless injuries may occur.
Threshold quantitative sensory testing is probably more reproducible than the subjective assessment by the patient of the strength of stimulus.
dysaesthesia (peripheral)
Defined as an unpleasant abnormal sensation of burning, tingling, and numbness, associated with peripheral nerve lesions.
reduced or absent ankle reflexes (peripheral)
Common finding on clinical examination.
painless injuries (peripheral)
May develop over pressure points, most commonly on the foot, over the metatarsal heads.[Figure caption and citation for the preceding image starts]: Plantar ulcer in a patient with type 1 diabetesFrom the collection of Dr Rodica Pop-Busui, University of Michigan [Citation ends].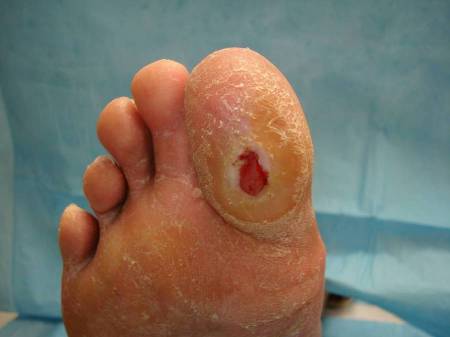
An object may become lodged in the shoe and erode through the skin with normal walking and weight-bearing.
Infection is a common complication, followed by gangrene if vascular dysfunction is present.
resting tachycardia (autonomic)
Resting tachycardia and a fixed heart rate are characteristic late findings in diabetic patients with vagal impairment. Resting heart rate is easy to measure at the bedside, but is not specific.
May be associated with exercise intolerance.
impaired heart rate variability (autonomic)
Could reveal early signs of cardiac autonomic neuropathy, but is challenging to measure correctly in clinical practice, and is currently used for clinical research only.
urinary frequency, urgency, nocturia, incontinence, hesitancy, weak stream, or retention (autonomic)
Associated with incomplete bladder emptying, increased post-void residual, lower peak urinary flow rate, bladder over-distension, and ultimately urinary retention and overflow incontinence.
Present in up to 50% of patients with diabetes.[99]
erectile dysfunction (autonomic)
Present in 30% to 75% of men with diabetes.[99] Can be the earliest symptom of diabetic autonomic neuropathy.
It is generally accepted that it is not solely induced by autonomic neuropathy, but rather by the co-existence of other multiple vascular risk factors, such as hypertension, hyperlipidaemia, obesity, and psychogenic factors.
decreased sexual desire and increased pain during intercourse (autonomic)
Women may present with sexual dysfunction characterised by decreased sexual arousal and inadequate lubrication.[73]
uncommon
orthostatic hypotension (autonomic)
Abnormal fall in systolic/diastolic BP in response to standing.
Easy to measure at bedside or clinic. Is usually a late characteristic and has low specificity.
The postural change in BP is elicited by a supine measurement followed by BP measurements at 1, 2, 3, and sometimes 5 minutes after standing.
About twice as common in people with type 1 diabetes as in those with type 2 diabetes.
Consequence of efferent sympathetic vasomotor denervation, causing reduced vasoconstriction of the splanchnic and other peripheral vascular beds.
May result in light-headedness, weakness, faintness, dizziness, visual impairment, and even syncope on standing.
Other diagnostic factors
common
constipation (autonomic)
Can alternate with episodes of diarrhoea.
faecal incontinence (autonomic)
May occur due to poor sphincter tone.
anhidrosis, heat intolerance, dry skin, or hyperhidrosis (autonomic)
May be complaints with sudomotor (sweating) dysfunction.
hypoglycaemia unawareness (autonomic)
May be triggered by autonomic neuropathy, but this relationship is complex.
uncommon
weakness (peripheral)
Less common than pain or loss of sensation, and is usually minor and occurs later.
May become clinically apparent with weakness of toe dorsiflexion and intrinsic hand muscles.
history of recent falls (peripheral)
May reflect gait and balance disorders associated with peripheral neuropathy.[65]
gait ataxia (peripheral)
Present in situations associated with severe peripheral denervation.
Especially obvious at night or when the patient walks with closed eyes.
nausea, postprandial vomiting, bloating, loss of appetite, early satiety (autonomic)
Associated with diabetic gastroparesis (delayed gastric emptying of solids or liquids, in the absence of mechanical obstruction).
Occurs as a consequence of dysfunction of the vagus nerve and intrinsic enteric autonomic nerves.
heartburn and dysphagia for solids (autonomic)
May be associated with oesophageal dysfunction, which results, at least in part, from vagal dysfunction.
profuse and watery diarrhoea (autonomic)
May be associated with diabetic gastroparesis. Typically intermittent and may occur at night.
Extremely difficult to treat.
Occurs in 20% people with diabetes and may alternate with constipation.[99]
specific mononeuropathy (peripheral)
Rare presentation of DN.
Presents with specific features depending on the nerve affected (e.g., carpal tunnel syndrome, or foot drop related to common peroneal neuropathy).
cranial neuropathy (peripheral)
Extremely rare presentation of DN.
More likely in older patients who have had diabetes for a long time.
pain over lower thoracic or abdominal wall (peripheral)
Feature of diabetic truncal radiculoneuropathy.
More common in men and tends to resolve within 4 to 6 months.
thigh muscle atrophy, pain, and weakness (peripheral)
Features of diabetic amyotrophy.
More common in type 2 diabetes.
Risk factors
strong
poorly controlled hyperglycaemia
The role of hyperglycaemia is supported by strong prospective randomised controlled trials and prospective observational studies.[10][11][15][30][33]
The annual incidence of DN was approximately 2% in conventionally treated patients with type 1 diabetes, but that rate dropped to 0.6% in intensively treated patients.[9]
prolonged duration of diabetes
older age (e.g., >70 years)
The incidence and prevalence of DN increase with age.[30]
There is a highly significant correlation between age and prevalence of neuropathy in both type 1 and type 2 diabetes.[5] However, neuropathy may be present in younger patients with type 2 diabetes and even at the diagnosis of type 2 diabetes.[27][48]
tall stature
hypertension
dyslipidaemia with elevated triglycerides
Elevated levels of low-density lipoprotein cholesterol and triglycerides are associated with an increased risk of DN.[53]
Evidence shows that elevated triglycerides are an important predictor of myelinated fibre density loss, independent of disease duration, age, diabetes control, or other variables.[37]
High levels of high-density lipoprotein cholesterol may reduce the risk of DN.[53]
co-existence of multiple cardiovascular disease (CVD) risk factors (type 2 diabetes)
In people with type 2 diabetes, co-existence of multiple CVD risk factors is associated with higher risk for DN.[54] Studies looking at the association between DN and metabolic syndrome have suggested that risk of DN in a patient may increase with increasing number of metabolic syndrome components (e.g., hypertriglyceridaemia, hypertension, abdominal obesity, low high-density lipoprotein levels).[55]
renal disease
Low estimated glomerular filtration rate and presence of macroalbuminuria or microalbuminuria have been associated with development of DN.[56]
weak
obesity
immune dysregulation
Serological signs of autoimmunity have been associated with development of diabetic peripheral neuropathy.[58]
There are reports of lymphocytic infiltration within autonomic nerve structures in diabetic patients with severe symptomatic autonomic neuropathy.[59] Auto-antibodies to autonomic nerve structures have also been reported.[60][61][62]
smoking
genetic
There is a genetic role in the development of DN. Single nucleotide polymorphisms that predispose to or are protective for diabetic peripheral neuropathy have been identified in metabolism and vasculature genes.[7]
Use of this content is subject to our disclaimer