Approach
Patient presentation can be very helpful in determining if symptoms are consistent with Creutzfeldt-Jakob disease (CJD) or if they can be attributed to another condition. An exclusionary approach should be used when diagnosing patients, although brain diffusion-weighted imaging (DWI) and attenuated diffusion coefficient map (ADC) MRI findings have high diagnostic utility.[65][66][67] Real-time quaking-induced conversion (RT-QuIC) of the CSF also has high diagnostic value.[68][69][70]
History
The onset of sporadic CJD (sCJD) is subacute, but overall progress is quite rapid relative to other slower-developing dementias such as Alzheimer's disease. Potential risk factors should be noted including certain medical procedures that the patient may have undergone (iatrogenic CJD) and overseas trip details (dates, duration, consumption of beef), particularly in the UK for variant CJD (vCJD).
Upwards of 60% of CJD patients found to carry a mutation in the prion protein gene (PRNP) were not known to have a family history of prion disease. Capturing a detailed family tree is important but it is recommended that all patients be tested for gene mutation even if there is no family history. The family history should be evaluated for dementia, other neurological conditions, and psychiatric disorders, as genetic forms of prion disease are often misdiagnosed as other conditions.
Neurological and physical examination
The clinical presentation of prion disease probably depends on the regions of the brain in which the prion is accumulating. Symptoms can mimic other neurological or psychiatric conditions. Patients suspected of having prion disease should be immediately referred to a neurologist. A detailed neurological examination is vital. Features found on examination will help direct the exclusionary work-up.
Most commonly, sCJD occurs in the seventh decade of life (patient in their 60s), and typically presents with the following.
Cognitive complaints, lack of co-ordination, behavioural and/or visual changes.[71]
In a study of more than 100 CJD cases, cognitive problems were the most common first symptom.[71] Patients frequently experience memory loss, aphasia, and difficulty with executive functioning (e.g., organising, planning, and multitasking).
Cerebellar, other motor, and behavioural symptoms were the next most common symptoms. Motor features include parkinsonism, myoclonus, and limb and/or gait ataxia. Involvement of the frontal lobes or frontal subcortical connections can affect behaviour causing agitation, depression, and other psychiatric features.[72]
Some patients may also describe having non-specific or constitutional symptoms such as vertigo, headaches, and dizziness that may precede the disease by weeks or even months.[73]
Visual symptoms are generally less common, but may include diplopia, hallucinations, and other visual distortions.[72]
vCJD presents quite differently from the sporadic form.
It typically affects young adults and teens.
In most patients, the first symptoms are psychiatric, including profound depression and mild cognitive impairment.
Later on in the course, patients develop dementia, ataxia, painful sensory symptoms, and/or a movement disorder.[11][21][71]
Genetic CJD is due to more than 40 different mutations in the prion gene (PRNP) and may be further subdivided into familial CJD, Gerstmann-Straussler-Scheinker (GSS), and fatal familial insomnia (FFI) based on clinical and pathological findings.
Familial CJD can have a longer, slower course than GSS or FFI.
The clinical course of GSS is typically longer and slower than that of sCJD - often for a few years and as long as a decade. Parkinsonism or ataxia may be the presenting signs of GSS, which can be misdiagnosed as other slower neurodegenerative conditions such as atypical parkinsonian dementias, Parkinson's disease, and multiple system atrophy.[74]
FFI typically presents as a syndrome of insomnia and dysautonomia. Ataxia or cerebellar incoordination can occur. Dementia occurs later in the course of the disease.[75]
MRI
MRI should be ordered as soon as a rapidly progressive dementia is suspected. Findings are especially valuable in the diagnosis of prion disease, and have a high sensitivity and specificity when using fluid-attenuated inversion recovery (FLAIR), and particularly diffusion-weighted imaging (DWI) or attenuated diffusion coefficient map (ADC) imaging.[66][67][76][Figure caption and citation for the preceding image starts]: Changes in the basal ganglia seen in Creutzfeldt-Jakob disease. (A) Fluid-attenuated inversion recovery MRI and (B) diffusion-weighted MRI of the same patient demonstrate bilateral basal ganglia hyperintensities (arrows). There is also mild bilateral medial thalamus and pulvinar hyperintensityFrom the personal collection of Dr M. Geschwind [Citation ends].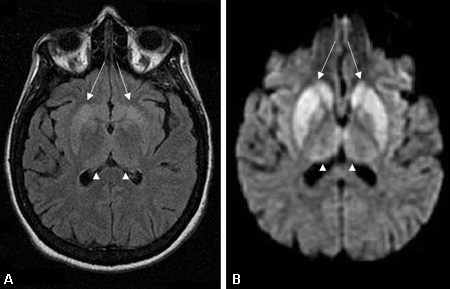 [Figure caption and citation for the preceding image starts]: Bilateral medial thalamus and pulvinar hyperintensity (arrowheads) on (A) fluid-attenuated inversion recovery and (B) diffusion-weighted MRI in a patient with Creutzfeldt-Jakob. This patient also has significant basal ganglia hyperintensity on both sequences (arrows)From the personal collection of Dr M. Geschwind [Citation ends].
[Figure caption and citation for the preceding image starts]: Bilateral medial thalamus and pulvinar hyperintensity (arrowheads) on (A) fluid-attenuated inversion recovery and (B) diffusion-weighted MRI in a patient with Creutzfeldt-Jakob. This patient also has significant basal ganglia hyperintensity on both sequences (arrows)From the personal collection of Dr M. Geschwind [Citation ends]. [Figure caption and citation for the preceding image starts]: Diffuse cortical ribboning (arrows) seen on (A) diffusion-weighted imaging (DWI) and less so on (B) fluid-attenuated inversion recovery (FLAIR) MRI. Both sequences show cerebral cortex gyral hyperintensitiesFrom the personal collection of Dr M. Geschwind [Citation ends].
[Figure caption and citation for the preceding image starts]: Diffuse cortical ribboning (arrows) seen on (A) diffusion-weighted imaging (DWI) and less so on (B) fluid-attenuated inversion recovery (FLAIR) MRI. Both sequences show cerebral cortex gyral hyperintensitiesFrom the personal collection of Dr M. Geschwind [Citation ends].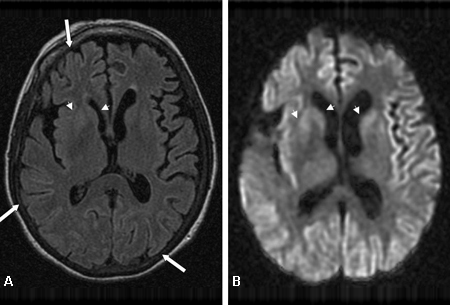
FLAIR and DWI MRI demonstrate hyperintensity in the cerebral cortex gray matter gyri, basal ganglia (caudate and putamen), and, less commonly, thalamus.[67][77]
On T1 images with gadolinium, CJD generally does not show contrast enhancement or white-matter intensity abnormalities. If these are found, other diagnoses should be investigated.[78][79] Some CJD patients have T1 hyperintensity in the globus pallidus.
The pulvinar sign, a term referencing bilateral pulvinar hyperintensity, can be seen in vCJD, and when both the pulvinar and medial thalamic show signal intensity, vCJD should be suspected.[71][80][81]
The ADC and DWI abnormalities found on MRI in sCJD and vCJD generally have not been reported in other similar dementias and are strongly suggestive of CJD.[67]
EEG
EEG is routine. Findings can include generalised slowing, focal or diffuse, and periodic sharp-wave complexes. These abnormalities, although moderately specific, are only about 60% sensitive and may not appear until the later stages of the disease.[77][82] When other conditions with similar EEG abnormalities have been ruled out, these findings can have high specificity for prion disease.[71]
CSF testing
The real-time quaking-induced conversion (RT-QuIC) is being used in a number of countries as a direct assay for the presence of abnormal prions in the CSF. It has very high specificity and sensitivity for CJD, particularly the sporadic forms.[68][69][70]
The 14-3-3 protein found in CSF was previously reported as being a strong indicator of CJD; however, the sensitivity and specificity of this test vary greatly in the literature.[71] Though there is great disagreement about the sensitivity of this test for sCJD, it is becoming more accepted in the neurology community that this test is not sufficiently specific for sCJD or other human prion diseases.[83] Current guidelines are shifting away from the use of this test.
Research studies have suggested that CSF proteins, such as total tau (T-tau) and neuron-specific enolase (NSE), have a slightly higher or equivalent sensitivity but much higher specificity than 14-3-3. However, these proteins can also be elevated in other rapidly progressive non-prion diseases, supporting the idea that they are released during neuronal death and injury and are not necessarily specific for prion disease.[84]
Although helpful in confirming rapid neuronal deterioration, these biomarkers cannot definitively diagnose or rule out prion disease.[66][71][83][85]
Blood and genetic testing
No approved blood tests are available for detecting prions, but patients should be screened for genetic mutations on the PRNP gene.
Samples will need to be sent to a specialist laboratory.
The National Creutzfeldt-Jakob Disease Research and Surveillance Unit Opens in new window
Public Health Agency of Canada: prion disease information Opens in new window
National Prion Disease Pathology Surveillance Center Opens in new window
It is recommended that patients and families undergo genetic counselling and understand the implications before undergoing testing and before learning genetic results. The genetic counselling protocol used for testing for Huntington's disease is typically followed for PRNP testing.[86]
Biopsy
Brain biopsy is the only definitive way to diagnose sporadic prion disease antemortem. In variant CJD, a tonsil biopsy can be diagnostic.
Due to the unpredictable pattern of protein accumulation in the brain, it is possible to get a false negative from brain biopsy. The procedure may place patients at unnecessary risk for infection or further brain damage. Even if the diagnosis is confirmed, there is still no available treatment.
Prion proteins are also resistant to standard surgical sterilisation methods, and medical staff performing the procedure may be placed at risk for CJD transmission. UK Department of Health guidance for minimising transmission risk is available.[27] Brain biopsy is only recommended when MRI is negative for CJD and all other conditions have been ruled out with other less-invasive methods.[71]
Autopsy
Autopsy is strongly encouraged, as pathological confirmation is the only definitive way to diagnose prion disease outside of biopsy.[36]
Use of this content is subject to our disclaimer