Recommendations
Urgent
Use an ABCDE approach to manage shock empirically. Treat the underlying cause as early as possible.
Ensure a patent airway. Get senior help immediately if you suspect airway compromise.[26]
Check for hypoxaemia and give oxygen if necessary .
Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[27]
A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[28]
Use mechanical ventilation if needed but beware this can worsen hypotension.[3]
Give intravenous fluids based on fluid status assessment. Use blood products and activate local major haemorrhage protocol if shock is secondary to acute bleeding.
Start with a fluid bolus of 500 mL normal saline or Hartmann’s solution and reassess need for further fluid in boluses of 250-500 mL.[30] Use smaller initial volumes (e.g., 250 mL) for patients with known cardiac failure or trauma.[26]
Avoid aggressive fluid resuscitation in cardiogenic shock as this can cause pulmonary oedema. Consider a loop diuretic if there is pulmonary oedema and fluid overload and/or a vasodilator if the patient has pulmonary oedema and systolic blood pressure >90 mmHg.
Give vasoactive drugs if not responding to intravenous fluids or blood products.
Do not use clear fluids in patients with shock secondary to acute bleeding (especially in trauma) unless blood products are not available.[31][94]
Look up your local major haemorrhage protocol as the ratio of these vary.[94]
UK National Institute for Health and Care Excellence (NICE) guidelines and British Society of Haematology (BSH) guidelines suggest a 1:1 ratio of red blood cells to fresh frozen plasma (FFP) in trauma and at least a 1:2 ratio in non-trauma patients.[31][94]
Key Recommendations
Definition
Shock is a life-threatening, generalised form of acute circulatory failure with inadequate oxygen delivery to, and consequently oxygen utilisation by, the cells.[2]
Treatment aims
The aims of treatment are to:
Give supportive treatment to reduce mortality
Treat the underlying cause.
Supportive management
Ensure a patent airway
Get senior help immediately if you suspect airway compromise.[26]
Use simple airway measures while waiting for help to arrive.[2]
Airway opening manoeuvres
Use head-tilt chin-lift if there is no risk of cervical spine injury
Use jaw thrust if there is risk of cervical spine injury.
Airway suction
Use to clear the airway if there is excess respiratory or gastric secretions.
Consider inserting an oropharyngeal or nasopharyngeal airway in deeply unconscious patients (Glasgow Coma Scale ≤8) [ Glasgow Coma Scale Opens in new window ]
Give oxygen if needed. Monitor controlled oxygen therapy. An upper SpO2limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia. A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[28]
Use non-invasive ventilation (NIV) if needed. Monitor blood pressure closely as the use of NIV in shocked patients can worsen hypotension.[37][109]
Target an initial mean arterial pressure (MAP) of 65 mmHg if the cause of shock is unknown.[1][2]
This target will change depending on the cause of shock and the patient’s normal blood pressure.[1][2]
Give intravenous fluids based on fluid status assessment unless shock is haemorrhagic.[30][31]
Use a crystalloid, either normal saline or Hartmann’s solution.
Start with a fluid bolus of 500 mL and reassess need for further fluid in boluses of 250-500 mL. Use smaller initial volumes (e.g., 250 mL) for patients with known cardiac failure or trauma.[26]
Give careful fluid resuscitation in cardiogenic shock as too much fluid can cause pulmonary oedema. Consider a loop diuretic and/or vasodilator.[3][29][36]
Give blood products in haemorrhagic shock. Use clear fluids if blood products are not available.[31][94]
Look up your local major haemorrhage protocol as the ratio of these vary.[94]
NICE guidelines and BSH guidelines suggest using a 1:1 ratio of red blood cells to FFP in trauma and at least a 1:2 ratio in non-trauma patients.[31][94]
Give vasoactive drugs (vasopressor/inotrope) if not responding to intravenous fluids or blood products.
Treat the underlying cause as early as possible
Escalate to a senior colleague if you suspect more than one type of shock (e.g., septic shock with subsequent anaphylactic shock secondary to administration of antibiotics) as this will require more complex management.
Distributive
Sepsis
Follow your local protocol for investigation and treatment of all patients with suspected sepsis, or those at risk. Start treatment promptly. Determine urgency of treatment according to likelihood of infection and severity of illness, or according to your local protocol.[24][110][111]
Think 'Could this be sepsis?' based on acute deterioration in an adult patient in whom there is clinical evidence or strong suspicion of infection.[24][112][113]
Use a systematic approach (e.g., National Early Warning Score 2 [NEWS2]), alongside your clinical judgement, to assess the risk of deterioration due to sepsis; urgently consult a senior clinical decision-maker (e.g., ST3 level doctor in the UK) if you suspect sepsis.[24][110][113][114] This review should take place:[24][110]
Within 30 minutes for a patient who is critically ill (e.g., NEWS2 score of 7 or more, evidence of septic shock, or other significant clinical concerns)
A patient is also at high risk of severe illness or death from sepsis if they have a NEWS2 score below 7 and a single parameter contributes 3 points to their NEWS2 score and a medical review has confirmed that they are at high risk.
Within 1 hour for any patient who is severely ill (e.g., NEWS2 score of 5 or 6) or within 1 hour of any intervention (antibiotics/fluid resuscitation/oxygen) if there is no improvement in the patient’s condition. Refer to or discuss with a critical care specialist or team.[24] Inform the responsible consultant.[24]
Refer to local guidelines for the recommended approach at your institution for assessment and management of the patient with suspected sepsis.
See Sepsis in adults.
Anaphylaxis
Give adrenaline (epinephrine) intramuscular injection to the mid-outer thigh. The dose can be repeated several times every 5 minutes (according to blood pressure, pulse, and respiratory function) if there is no improvement.[115]
Bear in mind that corticosteroids (e.g., hydrocortisone) are no longer advised for the initial emergency treatment of anaphylaxis.[47]
Antihistamines should not normally be used during initial emergency treatment of anaphylaxis. Non-sedating oral antihistamines, in preference to chlorphenamine, may be given following initial stabilisation especially in patients with persisting skin symptoms (urticaria and/or angioedema).[47]
See Anaphylaxis.
Cardiogenic
ST-elevation myocardial infarction (STEMI)[36][116]
Give all patients with suspected acute coronary syndrome a single loading dose of aspirin as soon as possible, unless they have aspirin hypersensitivity.[116]
As soon as a clinical diagnosis of STEMI has been made, seek immediate specialist input from the interventional cardiology team and commence urgent management.[36][116]
Immediately assess the patient's eligibility for coronary reperfusion therapy (irrespective of age, ethnicity, sex, or level of consciousness).[116]
For most patients the best option will be primary percutaneous coronary intervention (PCI); fibrinolysis is reserved for those without access to timely primary PCI.[36][116]
Rapid arrhythmias or severe bradycardia/conduction disturbance[3]
Give medical therapy, electrical cardioversion, or temporary pacing. This should be done urgently for patients with acute heart failure or haemodynamic instability.
Hypovolaemic
Haemorrhagic
Identify and aim to definitively stop the source of bleeding.[31]
Non-haemorrhagic
Diabetic ketoacidosis
Give a fixed-rate intravenous insulin infusion according to local protocols while monitoring blood glucose, potassium, sodium, ketones, and blood gases.[33]
Burns
Give fluid resuscitation guided by formal fluid resuscitation formulas. The Parkland formula is most commonly used in the UK.[117] [ Burn Injury Fluid Resuscitation, Adult (Parkland crystalloid estimate) Opens in new window ]
Obstructive
Cardiac tamponade
Consider pericardiocentesis or surgical drainage.[118]
See Cardiac tamponade.
Pulmonary embolism
Anticoagulate and thrombolyse if there are no contraindications.[106]
See Pulmonary embolism.
Tension pneumothorax
Check local protocols. Guidelines vary between recommending urgent decompression by needle thoracocentesis followed by a chest drain and performing a thoracostomy (rather than needle decompression) followed by chest drain insertion.[31][103]
See Tension pneumothorax.
Needle decompression of tension pneumothorax animated demonstrationHow to decompress a tension pneumothorax. Demonstrates insertion of a large-bore intravenous cannula into the fourth intercostal space in an adult.
When to escalate
Escalate all patients with shock to a senior clinician. In particular, escalate all complex patients such as those with:[30]
Pulmonary oedema
Severe sepsis
Hypernatraemia or hyponatraemia
Renal, liver, and/or cardiac impairment.
Escalate to critical care any patient with any of the following:[18][34]
Airway compromise
Severe hypoxaemia or need for non-invasive ventilation
Requirement for a vasopressor or inotrope
Significantly reduced consciousness (especially if Glasgow Coma Scale is ≤8)
Significant acidosis.
Monitoring
Repeat ABCDE to assess response to treatment.[1][2]
Reassess using thorough clinical examination and evaluation of vital signs every 30 minutes, which should include heart rate, blood pressure, oxygen saturations, respiratory rate, and temperature.
Monitor urine output.
Measure blood pressure via an arterial line if the patient fails to respond to initial treatment or needs vasoactive drugs. It provides precise, continuous monitoring, and access for arterial blood sampling.
Monitor lactate levels to help monitor response to treatment.[1]
The lactate level should decrease if the patient is clinically improving.
Frequency of repeat lactate measurement depends on cause of shock and treatment given.
Support airway, breathing, and circulation until the cause of shock is effectively treated.
Maintain a MAP to ensure adequate tissue perfusion and oxygenation.
Treat the underlying cause as soon as possible.
Get senior help immediately if you suspect airway compromise.[26]
This is an emergency, as airway obstruction causes hypoxia and risks damage to the brain, kidneys, and heart; cardiac arrest; and death.[26]
In the critically ill or shocked patient, depressed consciousness often leads to airway compromise.[26]
Practical tip
Conscious patients with airway compromise should be allowed to adopt whichever position they find most comfortable (typically sitting upright).
Use simple airway measures while waiting for help:[26]
Airway opening manoeuvres
Use head-tilt chin-lift if there is no risk of cervical spine injury.
Use jaw thrust if risk of cervical spine injury.
Airway suction
Generally used to clear the airway if there is excess respiratory or gastric secretions.
Consider inserting an oropharyngeal or nasopharyngeal airway in deeply unconscious patients (Glasgow Coma Scale [GCS] ≤8). [ Glasgow Coma Scale Opens in new window ]
Practical tip
Avoid endotracheal intubation and mechanical ventilation until the patient is adequately resuscitated, unless absolutely essential.[119]
Consider early intubation and ventilation for severe shock if there is respiratory distress, severe hypoxaemia, pronounced acidosis, or significantly reduced consciousness (GCS ≤8).[18][34] [ Glasgow Coma Scale Opens in new window ]
Intubation provides an unobstructed airway and protects against aspiration if there is a reduced level of consciousness.[34]
Practical tip
Seek senior critical care input at an early stage in profoundly shocked patients, particularly those with severe acidosis or impaired consciousness. These patients are likely to need early tracheal intubation.
Consider preloading with intravenous fluids and ensure that a vasopressor can be urgently administered if a patient is undergoing intubation. Induction in patients with severe shock may precipitate profound circulatory collapse. This is due to the myocardial depressant and vasodilating effects of many anaesthetic induction agents as well as the change to positive pressure ventilation when the patient is paralysed and mechanically ventilated.[34][18]
Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[27]
A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[28]
Evidence: Target oxygen saturation in acutely ill adults
Too much supplemental oxygen increases mortality.
Evidence from a large systematic review and meta-analysis supports conservative/controlled oxygen therapy versus liberal oxygen therapy in acutely ill adults who are not at risk of hypercapnia.
Guidelines differ in their recommendations on target oxygen saturation in acutely unwell adults who are receiving supplemental oxygen.
The 2017 British Thoracic Society (BTS) guideline recommends a target SpO2 range of 94% to 98% for patients not at risk of hypercapnia, whereas the 2022 Thoracic Society of Australia and New Zealand (TSANZ) guideline recommends 92% to 96%.[28][120]
The Global Initiative For Asthma (GINA) guidelines recommend a target SpO2 range of 93% to 96% in the context of a severe exacerbation of asthma.[121]
A systematic review including a meta-analysis of data from 25 randomised controlled trials, published in 2018, found that in adults with acute illness, liberal oxygen therapy (broadly equivalent to a target saturation >96%) is associated with higher mortality than conservative oxygen therapy (broadly equivalent to a target saturation ≤96%).[27] In-hospital mortality was 11 per 1000 higher for the liberal oxygen therapy group versus the conservative therapy group (95% CI, 2-22 per 1000 more). Mortality at 30 days was also higher in the group who had received liberal oxygen (relative risk 1.14, 95% CI 1.01 to 1.29). The trials included adults with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery. Studies that were limited to people with chronic respiratory illness or psychiatric illness, or patients on extracorporeal life support, receiving hyperbaric oxygen therapy, or having elective surgery, were all excluded from the review.
An upper SpO2 limit of 96% is therefore reasonable when administering supplemental oxygen to patients with acute illness who are not at risk of hypercapnia. However, a higher target may be appropriate for some specific conditions (e.g., pneumothorax, carbon monoxide poisoning, cluster headache, and sickle cell crisis).[122]
In 2019 the BTS reviewed its guidance in response to this systematic review and meta-analysis and decided an interim update was not required.[123]
The committee noted that the systematic review supported the use of controlled oxygen therapy to a target.
While the systematic review showed an association between higher oxygen saturations and higher mortality, the BTS committee felt the review was not definitive on what the optimal target range should be. The suggested range of 94% to 96% in the review was based on the lower 95% confidence interval and the median baseline SpO2 from the liberal oxygen groups, along with the earlier 2015 TSANZ guideline recommendation.
Subsequently, experience during the COVID-19 pandemic has also made clinicians more aware of the feasibility of permissive hypoxaemia.[124]
Management of oxygen therapy in patients in intensive care is specialised and informed by further evidence (not covered in this summary) that is more specific to this setting.[125][126][127]
Use non-invasive ventilation (NIV) unless contraindicated. Beware that use of NIV in shocked patients can worsen hypotension. Therefore, monitor blood pressure closely.[3][37][109]
NIV is contraindicated in the following:[37]
If pneumothorax is suspected
Confusion/agitation (especially if GCS ≤8)
Severe hypoxaemia
Copious respiratory secretions
Recent facial or upper airway surgery or in the presence of facial abnormalities such as burns or trauma
Fixed obstruction of upper airway
Vomiting.
Consider continuous positive airway pressure (CPAP) in:
Hypoxaemia requiring high respiratory rate, effort, and fraction of inspired oxygen (FiO 2)[3]
Left heart failure with severe dyspnoea and acidaemia at acute presentation or as an adjunct to medical therapy if the person's condition has failed to respond to improve hypoxaemia and cardiac output.[29]
Evidence: Use of CPAP in cardiogenic shock reduces mortality
Several studies have shown that CPAP is effective in patients with cardiogenic shock with acute heart failure as it rapidly improves gas exchange and cardiac haemodynamics, and can decrease intubation rates and in-hospital mortality.
One study randomised 40 patients to either face mask CPAP (10 cm H 2O) or standard medical therapy, and showed improvement in gas exchange, decrease in respiratory work, and reduced need for intubation for those patients with CPAP.[128] Another study randomised 55 patients to CPAP or high-flow oxygen therapy, and showed significant decrease in the intubation rates in the CPAP group compared with controls (28% vs 60%, respectively).[129]
A further comparison of the efficacy of CPAP (10 cm H 2O) with that of conventional treatment in a study of 39 patients with cardiogenic pulmonary oedema found a significant and rapid improvement in arterial oxygen tension and a significant decrease in arterial carbon dioxide tension in patients treated with CPAP compared with those treated conventionally. Whereas no patient needed endotracheal intubation in the CPAP group, 35% of the patients in the oxygen group were intubated within 3 hours of study entry. Although the final death rate was similar in both groups, patients given CPAP showed a significant reduction in intensive care unit length of stay.[130]
Consider bilevel positive airway pressure (BiPAP) if the patient has hypercapnic (type II) respiratory failure (PaCO2 >6 kPa or 45 mmHg and is acidotic [pH <7.35 or H+ >45 nanomol/L]), with targeted oxygen therapy if respiratory acidosis persists for more than 30 minutes after initiation of standard medical management.
Practical tip
Assess whether the patient needs intubation and invasive ventilation more than NIV. Do not hesitate to involve senior support.[37]
Escalate to critical care in any of the following:[37]
Impending respiratory arrest
NIV failing to augment chest wall movement or augment pCO 2
Inability to maintain target oxygen saturations on NIV
Need for intravenous sedation or presence of adverse features requiring closer monitoring.
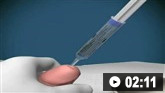
Establish early intravenous access in all patients.[31]
Use 2 wide-bore cannulas, especially in patients who are actively bleeding.
Consider intraosseous access if intravenous access is challenging.
Demonstrates how to obtain intraosseous access.
Practical tip
The intraosseous route can be used to administer most drugs and fluids, including blood products. However, they will not flow freely and drugs will need to be given by syringe, and blood/fluids need to be given through a pressure bag.
Target an initial MAP of 65 mmHg if the cause of shock is unknown.[1][2]
This target may change depending on the cause of shock and the patient’s normal blood pressure.[1][2]
Aim for a higher MAP in septic patients, patients with a history of hypertension, and those who show clinical improvement with higher blood pressure.[2]
Adjust target MAP for all patients with cardiogenic shock. No clinical studies have investigated the optimal blood pressure level, and current guidelines no longer recommend a target blood pressure.[2]
Tolerate a lower MAP in patients with uncontrolled bleeding until the source of bleeding is controlled unless they have a traumatic brain injury or spinal injury.[2][31]
Give fluid resuscitation in all patients except if there is evidence of acute pulmonary oedema. Patients with haemorrhagic shock need blood products.[1][3]
Give a fluid challenge.[2][3][30]
Use crystalloids starting with a bolus of 500 mL given over less than 15 minutes.[30]
Adjust according to patient’s characteristics such as age, size, and comorbidities.
In a patient with known cardiac failure, use smaller volumes (e.g., 250 mL).[26]
Do not give clear fluids to patients with hypovolaemic shock secondary to acute blood loss especially in trauma unless blood products are not immediately available.[31][39]
These patients need urgent identification and cessation of the source of bleeding and may need a blood transfusion or immediate transfer to theatre (e.g., in ruptured abdominal aortic aneurysm or major trauma).
Repeat fluid challenges based on frequent reassessment of haemodynamic status in boluses of 250-500 mL.[1][2][30]
Look for signs of hypovolaemia/fluid overload and assess the patient’s response to fluids. Possible outcomes include:
A sustained increase in blood pressure: proceed cautiously with fluids, then reassess in 15 to 30 minutes.
An increase and then a decrease in blood pressure: give another fluid bolus and reassess as patient appears fluid responsive
No change in blood pressure: means that either the patient is not fluid responsive, hypovolaemia is not the cause for the low blood pressure, or the patient has had adequate fluid replacement.
Practical tip
Fluid titration is essential in all patients (especially those with raised intravascular filling pressures or pulmonary oedema) as both hypovolaemia and hypervolaemia can be harmful.[2]
Check local protocols for specific recommendations on fluid choice. There is debate, based on conflicting evidence, on whether there is a benefit in using normal saline or balanced crystalloid in critically ill patients.
Practical tip
Be aware that large volumes of normal saline as the sole fluid for resuscitation may lead to hyperchloraemic acidosis.
Also note that use of lactate-containing fluid in a patient with impaired liver metabolism may lead to a spuriously elevated lactate level, so results need to be interpreted with other markers of volume status.
Evidence: Choice of fluids
Evidence from two large randomised controlled trials (RCTs) suggest there is no difference between normal saline and a balanced crystalloid for critically ill patients in mortality at 90 days, although results from two meta-analyses including these RCTs point to a possible small benefit of balanced solutions compared with normal saline.
There has been extensive debate over the choice between normal saline (an unbalanced crystalloid) versus a balanced crystalloid (such as Hartmann’s solution [also known as Ringer’s lactate] or Plasma-Lyte®). Clinical practice varies widely, so you should check local protocols.
In 2021-2022, two large double-blind RCTs were published assessing intravenous fluid resuscitation in intensive care unit (ICU) patients with a balanced crystalloid solution (Plasma-Lyte®) versus normal saline. The Plasma-Lyte 148 versus Saline (PLUS) trial (53 ICUs in Australia and New Zealand; N=5037) and the Balanced Solutions in Intensive Care Study (BaSICS) trial (75 ICUs in Brazil; N=11,052).[132][133]
In the PLUS study, 45.2% of patients were admitted to ICU directly from surgery (emergency or elective), 42.3% had sepsis, and 79.0% were receiving mechanical ventilation at the time of randomisation.
In BaSICS, almost half the patients (48.4%) were admitted to ICU after elective surgery and around 68% had some form of fluid resuscitation before being randomised.
Both found no difference in 90-day mortality overall or in prespecified subgroups for patients with acute kidney injury (AKI), sepsis, or post-surgery. They also found no difference in the risk of AKI.
In BaSICS, for patients with traumatic brain injury, there was a small decrease in 90-day mortality with normal saline - however, the overall number of patients was small (<5% of total included in the study) so there is some uncertainty about this result. Patients with traumatic brain injury were excluded from PLUS as the authors felt these patients should be receiving saline or a solution of similar tonicity.
A meta-analysis of 13 RCTs (including PLUS and BaSICS) confirmed no overall difference, although the authors did highlight a non-significant trend towards a benefit of balanced solutions for risk of death.[134]
A subsequent individual patient data meta-analysis included 6 RCTs of which only PLUS and BaSICS were assessed as being at low risk of bias. There was no statistically significant difference in in-hospital mortality (OR 0.96, 95% CI 0.91 to 1.02). However, the authors argued that using a Bayesian analysis there was a high probability that balanced solutions reduced in-hospital mortality, although they acknowledged that the absolute risk reduction was small.[135]
A prespecified subgroup analysis of patients with traumatic brain injury (N=1961) found that balanced solutions increased the risk of in-hospital mortality compared with normal saline (OR 1.42, 95% CI 1.10 to 1.82).
Previous evidence has been mixed.
A 2015 double-blind, cluster randomised, double-crossover trial conducted in four ICUs in New Zealand (N=2278), the 0.9% Saline vs Plasma-Lyte for ICU fluid Therapy (SPLIT) trial, found no difference for in-hospital mortality, acute kidney injury, or use of renal-replacement therapy.[136]
However, a 2018 US multicentre unblinded cluster-randomised trial - the isotonic Solutions and Major Adverse Renal events Trial (SMART), among 15,802 critically ill adults receiving ICU care - found possible small benefits from balanced crystalloid (Ringer’s lactate or Plasma-Lyte) compared with normal saline. The 30-day outcomes showed a non-significant reduced mortality in the balanced crystalloid group versus the normal saline group (10.3% vs. 11.1%; odds ratio [OR] 0.90, 95% CI 0.80 to 1.01) and a major adverse kidney event rate of 14.3% versus 15.4%, respectively (OR 0.91, 95% CI 0.84 to 0.99).[137]
A 2019 Cochrane review included 21 RCTs (N=20,213) assessing balanced crystalloids versus normal saline for resuscitation or maintenance in a critical care setting.[138]
The three largest RCTs in the Cochrane review (including SMART and SPLIT) all examined fluid resuscitation in adults and made up 94.2% of participants (N=19,054).
There was no difference in in-hospital mortality (OR 0.91, 95% CI 0.83 to 1.01; high-quality evidence as assessed by GRADE), acute renal injury (OR 0.92, 95% CI 0.84 to 1.00; GRADE low), or organ system dysfunction (OR 0.80, 95% CI 0.40 to 1.61; GRADE very low).
Cardiogenic shock
Give careful fluid resuscitation for patients in cardiogenic shock as too much fluid may cause or worsen acute pulmonary oedema.
These patients may need additional specialised monitoring of their cardiac function, cardiac output, and preload to determine fluid requirements.[2][3][14]
Consider a loop diuretic if there is evidence of pulmonary oedema and fluid overload.[3][29]
Intravenous furosemide is the most commonly used first-line diuretic.
Evidence: Loop diuretics are widely used but may cause adverse outcomes
There are a lack of high-quality data that support the safety and efficacy of loop diuretics in cardiogenic shock. Despite this, they are still widely used and recommended in major guidelines.[3][29]
Intravenous furosemide in patients with cardiogenic shock typically results in a prompt diuretic effect (within 30 minutes) that peaks at 1.5 hours. This leads to a decrease in ventricular filling pressures and improvement in symptoms in most patients. Two large randomised controlled trials showed this effect and that treatment based primarily on loop diuretics was associated with rapid and substantial improvement of dyspnoea.[139][140]
However, administration of loop diuretics activates the renin-angiotensin-aldosterone system and the sympathetic nervous system, both of which play a fundamental role in heart failure progression.[141][142][143]
Loop diuretics also significantly decrease glomerular filtration rate in some patients with heart failure, presumably due to renin-angiotensin-aldosterone system and sympathetic nervous system activation with related changes in renal blood flow and glomerular filtration pressure.[144]
Clinically, observational studies suggest an association between diuretic use and worsening outcomes in patients.[145][146] In a retrospective study of 6797 patients, use of a diuretic was associated with a 37% increase in the risk of arrhythmic death after controlling for multiple other measures of disease severity.[147]
Several other studies have identified an association between higher doses of diuretics and adverse outcomes in patients.[148][149]
If the patient has pulmonary oedema and systolic blood pressure >90 mmHg, consider a vasodilator (e.g., glyceryl trinitrate, nesiritide) and ensure the patient is transferred to a critical care environment so that blood pressure can be continuously monitored.[3][29][150]
Both intravenous glyceryl trinitrate and nesiritide lower left ventricular filling pressure and provide symptomatic improvement.[151]
Glyceryl trinitrate is the preferred drug.
Monitor blood pressure in these patients in a critical care environment.
Haemorrhagic shock
Activate local major haemorrhage protocol for all patients with haemorrhagic shock.
Do not use clear fluids for patients with active bleeding unless blood products are not available.[31]
Use blood products (red blood cells and FFP).[31][39][94]
Look up your local major haemorrhage protocol as the ratio of these vary.
NICE and BSH guidelines suggest using a 1:1 ratio of red blood cells to FFP in trauma and at least a 1:2 ratio in non-trauma patients.[31][94]
BSH guidelines also recommend further blood products in the following circumstances:
Give platelets if platelets <75 x 10⁹/L (or <100 x 10⁹/L in brain and spine injuries)
Give cryoprecipitate or fibrinogen concentrate if fibrinogen <1.5 g/L (150 mg/dL) (<2 g/L [<200 mg/dL] if obstetric)
Give FFP if prothrombin time and/or activated partial thromboplastin time is >1.5 times normal.
Target a haemoglobin (Hb) level of 7-9 g/dL (70-90 g/L).[18][39]
Consider a target Hb of 8-10 g/dL (80-100 g/L) in patients with ischaemic heart disease.
Reverse anticoagulation if a patient is taking an anticoagulant.
Give a prothrombin complex concentrate to reverse warfarin.[155]
Give idarucizumab to reverse dabigatran.[156]
Give andexanet alfa (recombinant coagulation factor Xa) to reverse apixaban and rivaroxaban.[157] Andexanet alfa is not available in the UK.
There is no licensed reversal agent for edoxaban as yet.
Give intravenous tranexamic acid in trauma patients. Treatment should commence within 3 hours of injury. Consider use in non-traumatic haemorrhage also.[31][94]
Practical tip
Do not be falsely reassured by a normal haemoglobin in an early blood sample. Vasoconstriction in acute blood loss delays a fall in haemoglobin and may mask the severity of haemorrhagic shock.
Manage patients who require a vasoactive drug (vasopressor/inotrope) in a critical care setting.
Insert an arterial line and central venous catheter (CVC) in patients with shock that is not responsive to initial therapy and/or requires infusion of a vasoactive drug.[2][29]
This allows continuous accurate blood pressure monitoring and regular blood gas analysis.
Selection of appropriate vasoactive agents should only take place under critical care supervision and may vary according to the type of shock, clinician preference, and local practice guidelines.
Give all vasoactive drugs through a CVC (with the exception of metaraminol and adrenaline [epinephrine], which can be given both peripherally and through a CVC) to minimise the risk of extravasation and subsequent tissue necrosis.
Adrenaline can be given as a bolus via peripheral access while preparing a noradrenaline (norepinephrine) infusion or obtaining central or arterial access. Ideally this should be through a large-bore cannula in the antecubital fossa. However, an adrenaline infusion should always be given via a CVC.
Use a vasopressor (e.g., noradrenaline) if there is hypotension or continuing reduced tissue hypoperfusion despite intravenous fluids.[1]
Vasopressors are particularly useful in high cardiac output, low peripheral resistance states.
Give them only after adequate fluid resuscitation. Use of vasopressors when the patient is fluid-deplete can worsen tissue perfusion.
Vasopressors cause vasoconstriction that aims to reverse the mismatch between vessel tone and intravascular volume.
They increase the risk of tissue ischaemia and necrosis in a dose-dependent manner. Other adverse effects include decreased cardiac output, increased risk of tachycardia and arrhythmias, and increased cardiac work.[158][159][160]
Use an inotrope (e.g., dobutamine) if there is evidence of impaired cardiac function and a low/inadequate cardiac output and if signs of tissue hypoperfusion persist after preload optimisation.[2]
Do not use for isolated impaired cardiac function without a low/inadequate cardiac output.
Do not use in patients with haemorrhagic shock.
Inotropes increase cardiac output by increasing both stroke volume and heart rate. This increases MAP and maintains perfusion to vital organs and tissues.
Adverse effects include arrhythmias, tachycardia, hypertension/hypotension, and anginal chest pain.[161]
Consult a specialist for guidance on suitable vasopressor/inotrope regimens.
Escalate to a senior if more than one type of shock is suspected (e.g., septic shock with subsequent anaphylactic shock secondary to administration of antibiotics) as this requires more complex management.
Distributive
Sepsis
Follow your local protocol for investigation and treatment of all patients with suspected sepsis, or those at risk. Start treatment promptly. Determine urgency of treatment according to likelihood of infection and severity of illness, or according to your local protocol.[24][110][111]
Think ' Could this be sepsis?' based on acute deterioration in an adult patient in whom there is clinical evidence or strong suspicion of infection.[24][112][113] See Sepsis in adults.
Use a systematic approach (e.g., National Early Warning Score 2 [NEWS2]), alongside your clinical judgement, to assess the risk of deterioration due to sepsis.[24][111][112][113][114]
Consult local guidelines for the recommended approach at your institution.
In the UK, arrange urgent review by a senior clinical decision-maker (e.g., ST3 level doctor) if you suspect sepsis.[24][110] This review should take place:[24]
Within 30 minutes for any patient who is critically ill (e.g., NEWS2 score of 7 or more, evidence of septic shock, or other significant clinical concerns).
A patient is also at high risk of severe illness or death from sepsis if they have a NEWS2 score below 7 and a single parameter contributes 3 points to their NEWS2 score and a medical review has confirmed that they are at high risk.
Within 1 hour for a patient who is severely ill (e.g., NEWS2 score of 5 or 6) or within 1 hour of any intervention (antibiotics/fluid resuscitation/oxygen) if there is no improvement in the patient’s condition. Refer to or discuss with a critical care specialist or team.[24] Inform the responsible consultant.[24]
See Sepsis in adults.
Anaphylaxis
Give adrenaline (epinephrine) by intramuscular injection to the mid-outer thigh. The dose can be repeated several times every 5 minutes (according to blood pressure, pulse, and respiratory function) if there is no improvement.[115]
Bear in mind that corticosteroids (e.g., hydrocortisone) are no longer advised for the initial emergency treatment of anaphylaxis.[47]
Antihistamines should not normally be used during initial emergency treatment of anaphylaxis. Non-sedating oral antihistamines, in preference to chlorphenamine, may be given following initial stabilisation especially in patients with persisting skin symptoms (urticaria and/or angioedema).[47]
See Anaphylaxis.
Cardiogenic
ST-elevation myocardial infarction (STEMI)
Give all patients with suspected acute coronary syndrome a single loading dose of aspirin as soon as possible, unless they have aspirin hypersensitivity.[116]
As soon as a clinical diagnosis of STEMI has been made, seek immediate specialist input from the interventional cardiology team and commence urgent management.[36][116]
Immediately assess the patient's eligibility for coronary reperfusion therapy (irrespective of age, ethnicity, sex, or level of consciousness).[36][116]
For most patients the best option will be primary percutaneous coronary intervention (PCI); fibrinolysis is reserved for those without access to timely primary PCI.[36][116]
Rapid arrhythmias or severe bradycardia/conduction disturbance
Correct severe rhythm disturbances urgently in patients with acute heart failure and unstable conditions with medical therapy, electrical cardioversion, or temporary pacing.[3]
Hypovolaemic
Haemorrhagic
Identify and aim to definitively stop the source of bleeding.[31]
Non-haemorrhagic
Diabetic ketoacidosis
Give a fixed-rate intravenous insulin infusion according to local protocols while monitoring blood glucose, potassium, sodium, ketones, and blood gases.[33]
Burns
Give fluid resuscitation guided by formal fluid resuscitation formulas. The Parkland formula is most commonly used in the UK.[117] [ Burn Injury Fluid Resuscitation, Adult (Parkland crystalloid estimate) Opens in new window ]
Obstructive
Cardiac tamponade
Consider pericardiocentesis or surgical drainage.[118]
See Cardiac tamponade.
Pulmonary embolism
Anticoagulate and thrombolyse if there are no contraindications.[106]
See Pulmonary embolism.
Tension pneumothorax
Check local protocols. Guidelines vary between recommending urgent decompression by needle thoracocentesis followed by a chest drain and performing a thoracostomy (rather than needle decompression) followed by chest drain insertion.[31][103]
See Tension pneumothorax.
Repeat ABCDE to assess response to treatment.[1][2]
Reassess with thorough clinical examination and evaluation of vital signs every 30 minutes, which should include heart rate, blood pressure, oxygen saturations, respiratory rate, and temperature.
Monitor urine output.
Measure blood pressure via an arterial line if the patient fails to respond to initial treatment or needs vasoactive drugs. It provides precise, continuous monitoring, and access for arterial blood sampling.
Monitor lactate levels to help monitor response to treatment.[1]
The lactate level should decrease if the patient is clinically improving.
Frequency of repeat lactate measurement depends on cause of shock and treatment given.
Ultrasound-guided insertion of a non-tunnelled central venous catheter (CVC) into the right internal jugular vein using the Seldinger insertion technique.
How to insert a peripheral venous cannula into the dorsum of the hand.
How to insert a urethral catheter in a female patient using sterile technique.
How to insert a urethral catheter in a male patient using sterile technique.
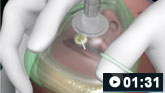
How to use a pocket mask to deliver ventilation breaths to an adult patient.
Use of this content is subject to our disclaimer