Recommendations
Urgent
Use an ABCDE approach to diagnose shock in order to treat empirically. Identify the underlying cause of shock as soon as possible to reduce mortality.
Check for hypotension[2]
Tachycardia may be an earlier sign of shock.[25]
Assess for inadequate tissue perfusion[1][2]
Check the skin for cool, clammy peripheries; a mottled, ashen appearance; and cyanosis of the skin, lips, or tongue.
Monitor urine output. Oliguria suggests poor renal perfusion.
Assess for changes in mental state. Agitation, confusion, and distress occur early; unresponsiveness indicates more severe and advanced shock.
Perform an arterial or venous blood gas.
Metabolic acidosis with raised serum lactate (>2 mmol/L [>18 mg/dL]) and negative base excess is always present in established shock.[25]
Assess severity of shock and resuscitate.
Ensure a patent airway. Get senior help immediately if you suspect airway compromise.[26]
Check for hypoxaemia and give oxygen if needed.
Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[27]
A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[28]
Use mechanical ventilation if needed but beware this can worsen hypotension.[3][29]
Give intravenous fluids based on assessment of fluid status (or blood products if shock is secondary to acute bleeding).[1][2][30][31]
Use a crystalloid, either normal saline or Hartmann’s.
Start with a fluid bolus of 500 mL and reassess need for further fluid in boluses of 250-500 mL. Use smaller initial volumes (e.g., 250 mL) for patients with known cardiac failure or trauma.[26]
Give vasoactive drugs if not responding to intravenous fluids or blood products.
Always measure temperature and glucose.
Identify and treat the underlying cause.[1][2][3][24]
Assess cardiac function if the cause of shock is not obvious after initial assessment.
Use point-of-care ultrasound as first-line investigation.
Key Recommendations
Definition
Shock is a life-threatening, generalised form of acute circulatory failure with inadequate oxygen delivery to, and consequently oxygen utilisation by, the cells.[1][2]
The term ‘shock’ describes a pathophysiological state with many different causes and is not a specific diagnosis.[2]
Identifying the shocked patient
It can be difficult to recognise shock. Patients look unwell and often have symptoms specific to the underlying cause (e.g., fever, chest pain, shortness of breath, or abdominal pain).
Clinical presentation
Clinical features can be non-specific but often include:
Systolic blood pressure of <90 mmHg or MAP of <65 mmHg, or a decrease of ≥40 mmHg from baseline.
Patients can be normotensive or hypertensive especially in the early stages of shock. Hypotension is not essential for the diagnosis. Tachycardia may be an earlier sign of shock.
Mottled, ashen, sweating and cyanosis of the skin, lips, or tongue. The skin may be cold or clammy peripherally.
Oliguria
Mental status changes[3]
Use the Glasgow Coma Scale (GCS). [ Glasgow Coma Scale Opens in new window ]
Agitation, confusion, and distress occur early, whereas unresponsiveness indicates more severe, advanced shock.
General features of shock include airway compromise, dyspnoea, hypoxaemia, and fever or hypothermia.[1][2][3][18][34]
Features due to the underlying cause may include chest pain in acute myocardial infarction or loin pain in ruptured abdominal aortic aneurysm.
Identify the underlying cause
It is essential to rapidly identify and treat the underlying cause to reduce mortality. Septic shock is the most common (62%), followed by cardiogenic (16%). There are four classes of shock:[2][3][30][33][35][36]
Distributive (e.g., sepsis, anaphylaxis, neurogenic, endocrine)
Cardiogenic (e.g., ST-elevation myocardial infarction, cardiomyopathy, arrhythmia)
Hypovolaemic (e.g., haemorrhage [trauma or internal bleeding such as ruptured aortic aneurysm, gastrointestinal bleeding], non-haemorrhagic [other fluid losses])
Obstructive (e.g., pulmonary embolism, tension pneumothorax, constrictive pericarditis).
Bear in mind that an individual patient may have more than one type of shock at the same time.[2]
Investigations
Perform the following in all patients with shock.[1][2][3][37][38][39]
Serum lactate level: >2 mmol/L (>18 mg/dL) is present in shock.
Arterial or venous blood gas: metabolic acidosis is present in shock.
Glucose: check for hyperglycaemia.
Blood tests including full blood count, urea and electrolytes, LFTs, coagulation studies, and CRP.
ECG: this should be continuously monitored. Look for tachyarrhythmias or bradyarrhythmias.
CXR: look for pulmonary oedema, pneumonia, pneumothorax, widened mediastinum (e.g., due to aortic dissection).
Point-of-care ultrasound: use a protocol such as Rapid Ultrasound in Shock and Hypotension (RUSH) to examine the lungs, inferior vena cava (IVC), aorta, and abdomen to look for the cause of shock. Assess cardiac function with echocardiography to identify the cause of shock, select the most appropriate treatment, and assess response to treatment.[14]
Use your initial assessment to guide further investigations into the cause of the shock.
When to escalate
Get senior help in all patients with shock. Escalate all complex patients such as those with:[30]
Sepsis
Cardiogenic shock
Pulmonary oedema
Hyper/hyponatraemia
Renal or liver disease.
Also escalate to critical care in patients with any of the following:[18][34]
Airway compromise
Severe hypoxaemia or need for non-invasive ventilation
Need for vasopressors or inotropes
Significantly reduced consciousness (especially if GCS is ≤8)
Significant hyperlactaemia (lactate >4 mmol/L [>36 mg/dL]).
Check for arterial hypotension, defined as a systolic blood pressure of <90 mmHg, or a mean arterial pressure (MAP) of <65 mmHg, or a decrease of ≥40 mmHg from baseline.[2]
Occurs in most patients but a normal blood pressure does not rule shock out.[3]
Blood pressure may be normal because compensatory mechanisms may preserve blood pressure through vasoconstriction, even though tissue perfusion and oxygenation are already decreased significantly.[2]
A low diastolic blood pressure suggests arterial vasodilation (such as in anaphylaxis or sepsis).[26]
A narrowed pulse pressure (the difference between systolic and diastolic pressures; normally 35–45 mmHg) suggests arterial vasoconstriction (such as in cardiogenic shock or hypovolaemia) and may occur with rapid tachyarrhythmias.[26]
Practical tip
Older patients can have shock at a normal systolic blood pressure of 110 to 120 mmHg due to atherosclerosis, hypertension, and less functional reflexes.[40]
Evidence: A normal blood pressure does not rule out shock
A systolic blood pressure of >95 mmHg is not a sensitive measure for ruling out moderate or significant blood loss in haemorrhagic shock.
The diagnostic accuracy of a systolic blood pressure of <95 mmHg associated with shock secondary to acute blood loss was assessed in a systematic review of physical findings in patients with hypovolaemia.[41] A random-effects model produced a sensitivity of 13% for moderate blood loss and 33% for severe blood loss. A decrease in cardiac output is associated with significant vasoconstriction, leading to decreased peripheral perfusion to maintain arterial pressure.[42]
Several studies show that a normal blood pressure can be associated with markers of inadequate tissue perfusion.[43][44]
These include decreased central venous oxygen saturation (ScvO 2) and significantly increased concentrations of blood lactate.
Persistent hypotension in patients with septic shock without increased lactate levels may only have limited impact on mortality.[45]
Tachycardia may be an earlier sign of shock than hypotension (due to compensatory mechanisms that maintain cardiac output).[25]
The baseline heart rate may be lower in:[24][25]
Young people and fit adults
Older people
If there is infection, they may not develop an increased heart rate
They may develop a new arrhythmia in response to shock (particularly in septic shock) rather than an increased heart rate
Patients who are taking certain medications that affect the heart rate response, such as beta-blockers.
The baseline heart rate may be higher in pregnancy.
This is usually 10-15 beats per minute more than normal.
Assess for signs of altered tissue perfusion
Cool, clammy peripheries
Mottled, ashen appearance and sweating
Cyanosis of skin, lips, or tongue.
Practical tip
In early sepsis the skin may be warm. This may become cooler if the patient deteriorates due to reduced peripheral perfusion.[46]
In anaphylaxis there may be patchy/generalised erythema, urticaria, and angio-oedema. These may be absent in 20% of patients with anaphylaxis.[47]
In neurogenic shock the skin may be warm and dry. Loss of sympathetic tone from spinal cord injury leads to vasodilation and no sweating.[35]
Oliguria[3]
Consider inserting a urinary catheter. Oliguria is <0.5 mL/kg/hour and is a sign of reduced renal perfusion.[2]
Reduced renal perfusion leads to reduced volume of concentrated urine with low sodium excretion. This is mediated by antidiuretic hormone and the renin-angiotensin-aldosterone system to retain sodium and water and correct the fluid depletion.[48]
Assess the patient’s mental status using the Glasgow Coma Scale. [ Glasgow Coma Scale Opens in new window ]
Agitation, confusion, and distress occur early. Unresponsiveness indicates severe and advanced shock.
Practical tip
Altered cognition is the most sensitive and universal sign of hypoperfusion.[2]
Assess for airway compromise[49]
Look for:
Swollen lips and tongue in anaphylaxis
Drooling and an inability to swallow secretions
Inflammation and sooty sputum following thermal/burns injury
Neck haematoma following blunt or penetrating injury
An associated rash in anaphylaxis and increased work of breathing seen in severe asthma
Abnormal chest and abdominal wall movement, suggesting airway obstruction
Lack of fogging of the oxygen mask.
Listen for:
Snoring noise of partial airway obstruction
Hoarse voice in anaphylaxis
Stridor or wheeze.
Gently feel for:
Unstable facial fractures
Fractures that could compromise the airway include maxillary or mandibular fractures, fractured or exfoliated teeth, and bleeding from epistaxis or facial fractures
Crepitus and surgical emphysema in laryngeal injury.
Practical tip
Get early senior critical care input in shocked patients, particularly with severe acidosis or impaired consciousness.
Look for signs of dyspnoea[3]
Look for tachypnoea and increased work of breathing.
Respiratory rate increases in hypoxia (e.g., in pneumonia) but will often stay elevated despite correction of PaO 2 as worsening perfusion generates a metabolic acidosis requiring respiratory compensation.[2]
Assess oxygen saturations[37]
Hypoxaemia may be secondary to:
Hypoxic hypoxaemia (e.g., pneumonia)
Stagnant hypoxaemia (e.g., cardiogenic shock)
Anaemic hypoxaemia (e.g., acute blood loss)
Cytotoxic hypoxaemia (e.g., carbon monoxide poisoning).
Give high-flow oxygen. Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Mechanical ventilation may be needed but can worsen hypotension.
Measure temperature[25]
Treat patients with hypothermia and shock early. Persistent hypothermia that is resistant to treatment is the most obvious clinical sign of end-stage irreversible shock of any cause.[25]
Hypothermia may be due to vasoconstriction and can also be seen in patients with trauma.[25]
Hyperthermia can also be present and can be due to many causes including sepsis, pulmonary embolism, certain toxins (e.g., salicylate overdose), and endocrine emergencies such as phaeochromocytoma or an addisonian crisis.[25][51]
Assess fluid status through history and physical examination in all patients with suspected shock.
Signs of hypovolaemia are:[30]
Reduced peripheral skin perfusion and skin temperature
Reduced skin turgor and dry mucous membranes.
Signs of fluid overload are:[30]
An elevated jugular venous pressure: this reflects venous return to the right atrium, and, therefore, hydration. However, it is also affected by cardiopulmonary disease. Right-sided cardiac failure, tricuspid regurgitation, and pulmonary hypertension also cause a rise in jugular venous pressure, even if the patient does not have fluid overload
A systolic murmur or third heart sound (functional mitral regurgitation)
Bibasal crepitations on chest auscultation: these occur with pulmonary oedema, but also in patients with pulmonary fibrosis, atelectasis, and infection (which sometimes leads to inappropriate diuretic therapy)
Dependent oedema (peripheral or sacral): usually indicates significant excess extracellular fluid
Hypertension (e.g., in patients with renal failure).
Use the passive leg-raising test if adequate monitoring is available.[2][30][52]
This is a useful indicator of fluid responsiveness that should be assessed using devices that can continuously monitor cardiac output in real time (e.g., Pulse index Continuous Cardiac Output [PiCCO] monitor or oesophageal Doppler) or serial transthoracic echocardiography, usually in an intensive care unit (ICU) rather than a general ward setting.
Sit the patient upright at 45° and tilt the entire bed through 45°.
Patients with a positive test have a >10% increase in cardiac output or stroke volume, indicating more fluids may be required.
Monitor cardiac function, cardiac output, and preload in shock to:[1][2][3]
Identify the type of shock if unclear on initial examination
Select the most appropriate treatment
Evaluate response to treatment.
Use echocardiography as a first-line investigation to identify the cause of shock if this is unclear on initial examination. Consider serial transthoracic echocardiography or invasive haemodynamic monitoring such as oesophageal Doppler (transpulmonary thermodilution is rarely used) in complex patients to determine the type of shock and response to treatment [1][2][53]
Distributive shock is characterised by an elevated cardiac output, while other types of shock are associated with low cardiac output.
Hypovolaemic shock is associated with low blood pressures and volumes, while these are increased in cardiogenic shock.
Obstructive shock is associated with increased pulmonary artery pressure and dilated right-sided cavities.
Cardiac tamponade, a form of obstructive shock, is associated with compression of all cavities, elevated intracardiac pressures, but small cardiac volumes. See Cardiac tamponade.
Determine fluid responsiveness (a measure of preload) to assess whether a patient requires additional fluid to increase cardiac output. Use dynamic assessments of cardiac function over static where possible [1][2]
Fluid responsiveness is defined as an increase of stroke volume of 10% to 15% after the patient receives 500 mL of crystalloid over 10-15 minutes.[2]
Static tests are less reliable than dynamic tests. They include:
Heart rate, blood pressure, collapsed veins, capillary refill time, urine output
Central venous pressure (CVP)
PiCCO[54]
This is a cardiac output monitor that combines pulse contour analysis and transpulmonary thermodilution techniques.
It provides an estimate of extravascular lung water, which is a sensitive indicator of pulmonary oedema.[55]
Dynamic tests are more useful but are limited by the fact that many techniques require the patient to be mechanically ventilated. They include:[2][30][56]
This can be used to:
Assess vena cava compressibility
If the IVC is compressible the patient may need more fluid
If the IVC is non-compressible the patient is unlikely to respond to more fluid.
Calculate the subaortic velocity time index, which allows measurement of stroke volume and cardiac output.
Assess the end diastolic volume, which can be used to estimate the preload.
Determine whether a patient is at risk of fluid overload and pulmonary oedema. A small IVC that varies in size with respiration, non-dilated right heart chambers, a non-displaced intra-ventricular septum, absence of right and left ventricular systolic failure, and absence of markers of raised left ventricular end-diastolic pressure all suggest that giving fluids will not cause acute harm.
Assessment of pulse pressure variation (PPV), stroke volume variation (SVV), and systolic pressure variation (SPV)[2][14]
These are measured using specialised haemodynamic monitors (such as PiCCO or LiDCO® devices).
Decide which parameter to use based on personal preference.
Practical tip
SVV of more than 12% accurately predicts fluid responsiveness with values over 14% having a very high positive predictive value and values less than 10% having a high negative predictive value.[57]
Assess cardiac output and stroke volume to determine whether inotropes should be used.[2]
Use echocardiography first-line to evaluate cardiac output and stroke volume.
Practical tip
Take into account the patient’s overall condition when using a measure of preload (particularly if it is a one-off measurement). A patient with a normal fluid status has a very low preload and does not require additional fluid. But some patients with high measures of preload may need additional fluids. It is better to evaluate changes in these parameters following interventions rather than use a single measurement.[2]
Evidence: Techniques used to determine cardiac function, cardiac output, and preload
Predicting fluid responsiveness with CVP and pulmonary artery occlusion pressure (PAOP) is controversial. Considerable evidence shows that they are not accurate measures of fluid responsiveness.[58]
In a study of normal healthy volunteers, both CVP and PAOP were shown to be poor predictors of preload, cardiac function, and changes in cardiac function following fluid loading compared with measurements of end-diastolic ventricular volumes. End-diastolic ventricular volumes have also been found to provide superior estimates of preload compared with CVP and PAOP in critically ill patients.[59]
The FENICE study, an observational study conducted in ICUs around the world, showed that static markers of preload are still used to test preload responsiveness in one third of instances.[60]
A systematic review identified 24 studies, which included 803 patients. Five studies compared CVP with measured circulating blood volume, while 19 studies determined the relationship between CVP and change in cardiac performance following a fluid challenge. The review showed a very poor relationship between CVP and blood volume as well as the inability of CVP to predict haemodynamic response to a fluid challenge.[61]
A retrospective study reviewed 96 patients with sepsis and concluded that CVP and PAOP are also poor predictors of fluid responsiveness in septic patients. A CVP of <8 mmHg and a PAOP of <12 mmHg predicted volume responsiveness with a positive predictive value of only 47% and 54%, respectively. When this was combined with low stroke volume index (<30 mL/m²), their positive predictive values were still unsatisfactory: 61% and 69%, respectively. When the combination of CVP and PAOP was considered instead of either pressure alone, the degree of prediction of volume responsiveness was not improved.[62]
Recent guidelines still state that a low CVP supports a response to fluid loading. The Surviving Sepsis Campaign guidelines suggest a CVP target of 8 mmHg to 12 mmHg in the first 6 hours (or 12 mmHg to 15 mmHg if mechanically ventilated) as initial resuscitation targets if a central line has been placed.[1]
Studies have shown that dynamic measures of fluid responsiveness are better predictors of fluid responsiveness than static parameters.
For example, PPV and SVV have been proven to be good predictors of fluid responsiveness in sedated, mechanically ventilated patients without spontaneous breathing activities and in sinus rhythm.[2] A meta-analysis that included 22 studies and 807 patients reported a pooled sensitivity for predicting fluid responsiveness with PPV of 88% and a specificity of 89%.[63]
Passive leg-raising has been shown to be helpful in predicting individual fluid responsiveness during spontaneous and positive pressure breaths while avoiding the risks of unnecessary fluid loading.[64][65]
Evidence: Evaluation of cardiac function to guide resuscitation
Evaluation of cardiac function is important in deciding whether to use inotropes in patients with shock. Inotropes should be given only when the altered cardiac function is accompanied by a low or inadequate cardiac output and signs of tissue hypoperfusion are present.[2]
In a trial involving more than 200 patients with septic shock, it was observed that several patients had a left ventricular ejection fraction (LVEF) of close to 40% even though their cardiac index was higher than 3 L/minute/m². Conversely, several patients had a low cardiac output but preserved cardiac function and inotropic stimulation should not be used in these patients.[66]
A prospective study of 46 patients with septic shock observed that echocardiographic assessment of myocardial function and preload responsiveness often led to different interventions than those guided by the resuscitation goals proposed by the Surviving Sepsis Campaign (SSC).[1][67] In this study, the authors found that agreement on the indication (or absence of indication) for inotropic administration occurred in 34 (74%) of the patients, but that the evaluation of LVEF suggested the use of inotropic agents in 11 patients for whom the SSC guidelines suggested otherwise. These authors therefore suggested that resuscitation should be guided by measurements of LVEF rather than by the SSC criteria. These data should, however, be interpreted with caution, as no analysis of patient outcome was performed.
Measure cardiac function and/or cardiac output to assess the response to fluids or inotropes.[2]
A change in cardiac output by at least 10% to 15% is a positive response to fluids.[2]
Use serial echocardiography to evaluate response to treatment.[2]
Consider using advanced haemodynamic monitors (such as PiCCO or LiDCO® devices) in complex shock states.
Insert an arterial line and central venous catheter (CVC) in all patients with shock who are not responding to fluid resuscitation or who need vasopressors.[2]
An arterial line allows continuous blood pressure monitoring and regular blood gas analysis.
A CVC is required for advanced haemodynamic monitoring using a PiCCO device. This allows aggressive rehydration in patients with cardiac disease without causing pulmonary oedema.
Give all vasoactive drugs via a CVC (with the exception of metaraminol and adrenaline [epinephrine]).
A CVC can also measure ScvO 2. This reflects the balance of oxygen delivery compared with oxygen consumption.
ScvO 2 <65% suggests impaired tissue oxygenation
ScvO 2 >80% suggests a high PaO 2, or suspect:
Impaired utilisation of oxygen by cells (e.g., severe sepsis)
Microcirculatory shunting (e.g., severe sepsis, liver failure)
Left-to-right shunts.
Practical tip
An important limitation of CVC-measured CVP is that atrial filling pressures are 'inappropriately' high in right-sided cardiac failure, tricuspid regurgitation, cor pulmonale, and in the presence of pulmonary embolism, even when these patients are hypotensive and oliguric and may need fluid replacement. Mechanical ventilation also raises CVP.[2]
Identify the possible underlying cause of shock through history and clinical examination. Do not delay supportive management if the cause is unclear, however.
There are four different categories of shock: distributive (failure of vasoregulation); cardiogenic (pump dysfunction); hypovolaemic (loss of intravascular volume); obstructive (barriers to cardiac flow or filling). Bear in mind that an individual patient may have more than one type of shock at the same time.[2]
Distributive (failure of vasoregulation)
Sepsis[1][24]
Identify possible source of infection through the history (e.g., cough, recent surgery, abdominal pain, dysuria).
Look for a non-blanching rash of the skin.
Check for breach of skin integrity (e.g., cuts, burns, ulcers, skin infections, swelling or discharge at surgical wound site). Remove any bandages.
Check temperature: there may be fever OR hypothermia.
See Sepsis in adults.
Practical tip
Consider sepsis in all patients, especially those presenting with signs or symptoms that indicate possible infection.[24]
Patients may not have an obvious source of infection. They may describe feeling very unwell and may not have a high temperature.[24]
Assess patients who might have sepsis with extra care if they cannot give a good history (for example, people with English as a second language or people with communication problems such as learning disabilities or autism).[24]
Anaphylaxis[47]
There may be a history of triggers (e.g., food, drugs, and stinging insects).
Assess for stridor or bronchospasm.
Auscultate the chest to listen for wheeze.
The patient may have a hoarse voice.
Look for flushing, urticaria (the weals may be pale, pink, or red, and may look like nettle stings and are usually itchy), and angio-oedema (most commonly in the eyelids and lips, and sometimes in the mouth and throat).
There may be gastrointestinal symptoms such as abdominal pain, vomiting, or incontinence.
See Anaphylaxis.
Neurogenic[35]
Usually indicated by an initially flaccid paralysis below the level of the lesion, with a history compatible with spinal trauma or a spinal lesion.
The bladder may be palpated due to urinary retention.
Cardiogenic (pump dysfunction)
There may be clues in the history such as chest pain, shortness of breath, syncope, palpitations, or nausea and vomiting.[3]
Myocardial infarction is the most common cause of left ventricular failure causing cardiogenic shock (75% to 80% cases).[25][36]
Other causes include tachyarrhythmias (e.g., atrial fibrillation or ventricular tachycardia), bradyarrhythmias, toxic substances (e.g., alcohol, recreational drugs), non-adherence with salt/fluid intake or medications, excessive rise in blood pressure, infection (e.g., pneumonia, infective endocarditis, sepsis), and acute mechanical causes (e.g., myocardial rupture, chest trauma, acute valvular incompetence).[3]
Assess for symptoms and signs of heart failure such as bilateral peripheral oedema, raised jugular venous pressure, orthopnoea, congested hepatomegaly, or hepatojugular reflex.[3]
Hypovolaemic (loss of intravascular volume)
Haemorrhagic
Gastrointestinal bleeding and trauma are common causes of haemorrhagic shock.[18][19] Other causes include:
Ruptured abdominal aortic aneurysm
Spontaneous bleeding from anticoagulation
Postpartum bleeding secondary to placenta praevia or placental abruption, and ruptured ectopic pregnancy or ruptured ovarian cyst.
Check for melaena if gastrointestinal bleeding is suspected.
Look thoroughly for external haemorrhage from wounds or drains or evidence of concealed haemorrhage (e.g., thoracic, intraperitoneal, retroperitoneal, or into gut).[26]
Practical tip
Intrathoracic, intra-abdominal, or pelvic blood loss may be significant following surgery, even if drains are empty.[26]
Always order a pregnancy test in a woman of childbearing age with shock. A ruptured ectopic pregnancy or a ruptured ovarian cyst can cause haemorrhagic shock without an obvious source of blood loss.
Perform a primary survey in trauma patients. Major sites of internal bleeding are the chest, abdomen, pelvis, and long bones.[18]
Practical tip
A useful mnemonic to remember the sites of major bleeding in trauma is ‘blood on the floor (external bleeding) and four more (internal bleeding - chest, abdomen, pelvis, and long bones)'.
Fractures of the pelvis can hide massive amounts of bleeding. If the pelvis is unstable, suspect significant blood loss.[68]
Spontaneous bleeding into the retroperitoneum can cause shock without significant physical findings.[68]
Fractures of the lower extremities, especially closed femur fractures, can easily hide 2–3 units of blood.[68]
Head injury is rarely a cause of hypotension and is never the cause of massive blood loss, unless there is external bleeding.[68]
Non-haemorrhagic
Burns
Diabetic ketoacidosis (DKA)
Look for the triad of ketonaemia, hyperglycaemia, and acidosis.[33]
Obstructive (barriers to cardiac flow or filling)
Pulmonary embolism (PE)
Acute dyspnoea, pleuritic chest pain, or features of deep vein thrombosis occur in 97% of patients with PE.[69]
Shock in PE indicates a massive (high-risk) PE.[70]
See Pulmonary embolism.
Cardiac tamponade
Often presents with dyspnoea, tachycardia, and chest pain.
Listen for muffled heart sounds and a pericardial friction rub (present in 50% but may be transient).[71]
See Cardiac tamponade.
Tension pneumothorax
Assess for ipsilateral hyperinflation of the hemithorax with diminished breath sounds, and hyper-resonance on percussion. There may be tracheal deviation towards the contralateral hemithorax.[72]
See Tension pneumothorax.
Evidence: Septic shock is the most common cause of shock
Septic shock was the most common cause of shock in 1679 ICU patients in the European Sepsis Occurrence in Acutely Ill Patients II (SOAP II) trial, accounting for 62% of cases, followed by cardiogenic shock (17%) and hypovolaemia (16%).[4]
Septic shock has case-fatality rates of 40% to 50%, reaching up to 80%.[73] There are limited data on the epidemiology of septic shock, particularly in low-income countries, but its incidence seems to be increasing.[74][75][76] Between 6.3% to 14.7% of admissions to ICU are for septic shock.[2][77]
Cardiogenic shock has most commonly been studied in the setting of acute myocardial infarction; the incidence in this population remains fairly constant at between 6% and 9%.[9][10] In a multinational observational study of 65,119 patients hospitalised for an acute coronary syndrome between 1999 and 2007, 4.6% developed cardiogenic shock, and the in-hospital case-fatality rate was 59.4%.[78] Anaphylaxis is rarely fatal and causes about 20 deaths/year in the UK.[79]
Always order
Lactate (from arterial blood gas)
Typically >2 mmol/L (>18 mg/dL) if shock is present [1][2][3]
Perform serial measurements if the lactate is >2 mmol/L (>18 mg/dL).
Lactate level should decrease if the patient is clinically improving.
Frequency of repeat testing depends on cause of shock and treatment given.
Increased serum lactate was previously thought to indicate tissue hypoperfusion, decreased tissue oxygenation, and anaerobic metabolism.[25] More recently, this is being challenged with the view that lactate is elevated due to the stress response, as anaerobic metabolism is a pre-terminal event.[32]
Practical tip
Involve senior support if lactate levels remain persistently >4 mmol/L (>36 mg/dL) as this indicates a high risk of death.[25]
Evidence: Measurement of lactate
Use serial measurement of lactate to guide resuscitation.
Five randomised controlled trials (647 patients) have evaluated lactate-guided resuscitation of patients with septic shock.[80][81][82][83][84] They showed a significant reduction in mortality in lactate-guided resuscitation compared with resuscitation without lactate monitoring. Two other meta-analyses of the 647 patients who were enrolled in these trials demonstrated moderate evidence for reduction in mortality when an early lactate clearance strategy was used, compared with either usual care or with treatment guided by ScvO 2 measurement.[85][86]
Evidence has also shown the value of serial lactate measurements for predicting prognosis.
One prospective study identified 76 patients with hypovolaemic shock and showed that changes in lactate concentrations provide an early and objective evaluation of a patient's response to therapy. It suggested that repeated lactate determinations represent a reliable prognostic index for patients with circulatory shock.[87]
Another observational study in patients with shock secondary to multiple trauma evaluated the correlation between lactate clearance and survival. All patients in whom lactate levels returned to the normal range (≤2 mmol/L [≤18 mg/dL]) within 24 hours survived. Survival decreased to 77.8% if normalisation occurred within 48 hours and to 13.6% in those patients in whom lactate levels were elevated above 2 mmol/L [18 mg/dL] for more than 48 hours.[88]
Venous blood gas (VBG) or arterial blood gas (ABG)
Use to detect metabolic acidosis with a high lactate and negative base excess, most commonly present in shock.[25][37]
VBG is increasingly being used as it is less invasive and painful.
Evidence suggests that venous pH has sufficient agreement with arterial pH for it to be an acceptable alternative in clinical practice for most patients.
Evidence: Base deficit as a prognostic marker
The initial base deficit on ABG is a good independent predictor of mortality in patients with haemorrhagic shock.
One study stratified the extent of base deficit into three categories of mild (-3 to -5 mEq/L), moderate (-6 to -9 mEq/L), and severe (<-10 mEq/L) and found a significant correlation between the admission base deficit and transfusion or fluid requirements within the first 24 hours and the risk of organ failure or death.[89]
The base deficit is a better prognostic marker of death than the pH in arterial blood gas analyses.[90]
Furthermore, the base deficit represents a highly sensitive marker for the extent of shock and mortality, both in adult and paediatric patients.[91][92]
Glucose
May be increased due to stress response initiated due to shock.[32]
A glucose level >7 mmol/L (>126 mg/dL) is abnormal in a non-diabetic patient.
May be increased in other causes such as diabetic ketoacidosis.[33]
Evidence: Hyperglycaemia is a response to critical illness
Stress hyperglycaemia and insulin resistance are evolutionarily preserved responses that allow the host to survive during periods of severe stress.[32]
In animal models of haemorrhagic shock the administration of hypertonic glucose solution increased cardiac output and blood pressure, and improved survival.
In these experiments, similar osmolar doses of saline or mannitol, with greater accompanying fluid volumes, failed to produce the sustained blood pressure changes or to improve the survival.[93]
Full blood count
Haemoglobin (Hb) may be decreased with acute bleeding[39][94]
Hb <100 g/L (<10 g/dL) is suggestive of haemorrhage as the cause; however, it may be normal in the early stages due to vasoconstriction.
Practical tip
Haemoglobin does not immediately fall in acute blood loss. Therefore, do not be falsely reassured with a normal Hb, especially if the blood sample has been taken early.
WBC may be increased with any cause of infection or inflammation.[24]
May be >12 x 10³/microlitre if sepsis is present.
Urea and electrolytes
Use to detect:[95]
Evidence of renal impairment if kidney perfusion is compromised.
Urea disproportionately raised with upper gastrointestinal bleeding, dehydration, or cardiac failure.[48]
Hyperkalaemia in trauma, acute kidney injury, DKA, and adrenal insufficiency.
Hypokalaemia with diarrhoea or vomiting.
Hypernatraemia in burns and diarrhoea or vomiting.
Hyponatraemia in trauma and also sometimes in diarrhoea and vomiting.
Coagulation studies
Use as a baseline test, especially prior to central venous catheter insertion.[2]
May also be deranged in patients with trauma and is a predictor of mortality.[38]
May also be deranged in disseminated intravascular coagulation secondary to sepsis.
Evidence: Coagulopathy is common in trauma patients with shock
The incidence of coagulation abnormalities, early after trauma, is high, and they are independent predictors of mortality even in the presence of other risk factors.
One study prospectively collected data on trauma patients presenting to a level 1 trauma centre. A logistic regression analysis was performed of prothrombin time (PT), partial thromboplastin time (PTT), platelet count, and confounders to determine whether coagulopathy is a predictor of all-cause mortality. An initial abnormal PT increased the adjusted odds of dying by 35% and an initial abnormal PTT increased the adjusted odds of dying by 326%.[38]
C-reactive protein
High C-reactive protein levels (>1904.8 nanomol/L [>200 mg/L]) indicate severe inflammation.
The higher the level, the greater the degree of inflammation.
Lower concentrations (<1904.8 nanomol/L [<200 mg/L]) may be found in septic states but also after myocardial infarction or surgery.
Procalcitonin (PCT)
Elevated PCT levels have been associated with sepsis and may help differentiate sepsis from causes of the systemic inflammatory response syndrome.[96][97] High PCT levels are associated with mortality from sepsis in 90-day follow-up. Other pro-inflammatory states, such as acute pancreatitis, trauma, major surgery, and burns, can also increase procalcitonin.[98] Changes in procalcitonin levels may occur later than that of lactate, although changes in both markers combined are highly predictive of outcome between 24 to 48 hours.[99]
ECG
May show evidence of:[3][14][36][69]
Myocardial ischaemia in cardiogenic shock
Right heart strain in massive PE
Underlying electrolyte abnormalities (e.g., hypokalaemia or hyperkalaemia).
Consider repeat ECGs or continuous monitoring of the ECG trace if patient is critically unwell. Ensure all patients with cardiogenic shock have continuous cardiac monitoring.[14]
Use to look for the underlying cause
Chest x-ray
Do not use for tension pneumothorax - urgent decompression is the first-line intervention. See Tension pneumothorax.
How to decompress a tension pneumothorax. Demonstrates insertion of a large-bore intravenous cannula into the fourth intercostal space in an adult.
Specifically consider:[24][103]
Consolidation in sepsis secondary to pneumonia
Pleural effusion in acute heart failure secondary to myocardial infarction
Pneumothorax
Pulmonary infarction secondary to PE
Widened mediastinum with aortic dissection.
Urinalysis and urine pregnancy test
Use to look for signs of infection in suspected sepsis.
Perform in all women of childbearing age to detect ectopic pregnancy.
Infection screen
Order if you suspect infection, especially in sepsis.
Decide which tests to order based on source of infection suspected. Specific tests include:
Blood cultures
Respiratory swabs for polymerase chain reaction
Sputum, urine, cerebrospinal fluid, or wound samples for microscopy, culture, and sensitivity.
Point-of-care ultrasound
Should only be performed by a senior doctor with specialist ultrasound training.
Follow a protocol such as Rapid Ultrasound in Shock (RUSH) to identify the underlying cause of shock by assessing the following.[104]
Lungs: B-lines suggest pulmonary oedema; lung sliding suggests pneumothorax.
Inferior vena cava: compressibility suggests the patient needs more fluid; non-compressibility suggests the patient may not respond to more fluid.
Abdominal aorta: abdominal aortic aneurysm.
Abdomen: ectopic pregnancy.
Use echocardiography to assess cardiac function in all patients with cardiogenic shock and undifferentiated shock.[14]
There is no benefit to ordering an echocardiogram in patients with haemorrhagic, anaphylactic, or neurogenic shock.
Do not routinely use in patients in whom the cause of shock is clear and who are responding to initial treatment.[2]
Use to:
Identify cause of shock if not clear on initial assessment
Left ventricular failure suggests myocardial infarction; right ventricular strain suggests PE; pericardial effusion suggests cardiac tamponade.
Select the most appropriate treatment
Use to evaluate cardiac output and stroke volume to determine whether inotropes are needed.
Assess response to treatment
Use to assess fluid responsiveness: defined as an increase of stroke volume of 10% to 15% after the patient receives 500 mL of crystalloid over 10-15 minutes.
Use Focused Assessment with Sonography in Trauma (FAST) in patients with suspected trauma to the chest, abdomen, or pelvis to look for free fluid.[105]
Computed tomography (CT) chest, abdomen, and pelvis
Use especially in patients with major trauma.[31]
Other indications may include suspected ruptured aortic aneurysm or intra-abdominal collection.
Potentially unsafe in a haemodynamically unstable patient. Patients must be stable before transfer to the imaging suite.
Computed tomographic pulmonary angiography (CTPA)
If you suspect pulmonary embolism (PE), use CTPA (if indicated) alongside other appropriate assessment tools.[106][107]
CTPA is the preferred investigation for definitive confirmation of PE; it is appropriate to use CTPA in most (but not all) patients.[106]
See Pulmonary embolism.
X-ray long bones
Use to detect fractures of the femur, which can cause shock due to blood loss, especially in older patients.
X-ray spine
Perform where there is suspicion of spinal injury in cases of neurogenic shock.
How to take a venous blood sample from the antecubital fossa using a vacuum needle.
How to record an ECG. Demonstrates placement of chest and limb electrodes.
How to obtain an arterial blood sample from the radial artery.
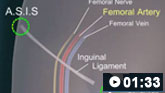
How to perform a femoral artery puncture to collect a sample of arterial blood.
Use of this content is subject to our disclaimer