Management of symptoms related to carcinoid syndrome is based on reducing hormonal secretion. Tumours normally secrete biogenic amines into the circulation. However, when the primary site is within the gut, the secreted amines are degraded by the liver and symptoms do not generally occur. When liver metastases are present, these amines drain into the circulation prior to being broken down and hence cause carcinoid syndrome.
Nearly all patients with carcinoid syndrome have liver metastases and many have unresectable disease. Consequently, symptomatic medical management is the initial mainstay of treatment.[22]Riechelmann RP, Pereira AA, Rego JF, et al. Refractory carcinoid syndrome: a review of treatment options. Ther Adv Med Oncol. 2017 Feb;9(2):127-37.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5298401
http://www.ncbi.nlm.nih.gov/pubmed/28203303?tool=bestpractice.com
Surgery, embolisation of metastases, radiofrequency ablation, or radiolabelled octreotide is appropriate in some patients.
Surgical management
Curative surgical therapy should always be considered. However, most patients have advanced disease. Surgery is an option in patients with a resectable primary lesion (e.g., a bronchial carcinoid tumour without evidence of metastatic spread). Surgery should be considered when both the primary and secondary lesions can be resected. Prior to surgery, patients should be commenced on octreotide infusion to prevent carcinoid crisis. It is commenced at least 2 hours prior to surgery and given until 48 hours after surgery.[23]Kaltsas G, Caplin M, Davies P, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: pre- and perioperative therapy in patients with neuroendocrine tumors. Neuroendocrinology. 2017;105(3):245-54.
https://www.doi.org/10.1159/000461583
http://www.ncbi.nlm.nih.gov/pubmed/28253514?tool=bestpractice.com
Bronchial carcinoid tumours: a significant number of these cases present with localised tumours and, thus, curative resection of the primary tumour is an option. Surgery should be considered as first-line therapy in those with a good functional status.[24]Soga J, Yakuwa Y. Bronchopulmonary carcinoids: an analysis of 1,875 reported cases with special reference to a comparison between typical carcinoids and atypical varieties. Ann Thorac Cardiovasc Surg. 1999 Aug;5(4):211-9.
http://www.ncbi.nlm.nih.gov/pubmed/10508944?tool=bestpractice.com
The type of surgery undertaken depends on the location, size, and extent of the tumour. In well-circumscribed lesions, wedge resection can be performed; other cases may require lobectomies and, occasionally, pneumonectomy. There is a low operative mortality of <1% for published surgical series, and the benefit is a possible curative resection.[24]Soga J, Yakuwa Y. Bronchopulmonary carcinoids: an analysis of 1,875 reported cases with special reference to a comparison between typical carcinoids and atypical varieties. Ann Thorac Cardiovasc Surg. 1999 Aug;5(4):211-9.
http://www.ncbi.nlm.nih.gov/pubmed/10508944?tool=bestpractice.com
Complications of surgery after initial resection relate to the type of procedure performed and previous functional status. Wedge excision and lobectomy leave a good residual lung volume, but pneumonectomy may impair quality of life post-operatively.
Midgut tumours: occasionally midgut carcinoid tumours without or with limited liver involvement are suitable for curative resection. Midgut carcinoids involving the small bowel should be resected if the patient is fit enough to undergo surgery. Occasionally, these patients present with small bowel obstruction and require emergency surgery. Resection of liver metastases can rectify hormonal markers and resolve symptoms. The type of surgery performed depends on the location, size, and number of liver lesions. Other than resection, radiofrequency ablation may also be performed and may sometimes be used at the time of surgery.
Debulking surgery for liver disease should be considered as a palliative option in patients with symptoms related to carcinoid syndrome refractory to medical therapy or in whom there is evidence of clinical/radiological progression of disease. In these cases, if resection of >90% of the tumour load is possible, then surgery may provide better symptom control and possibly longer survival.[25]Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000 Apr;190(4):432-45.
http://www.ncbi.nlm.nih.gov/pubmed/10757381?tool=bestpractice.com
[26]Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995 Jan;169(1):36-42.
http://www.ncbi.nlm.nih.gov/pubmed/7817996?tool=bestpractice.com
Medical management
Symptoms (flushing, diarrhoea, wheeze) are usually controlled with somatostatin analogues and interferon alfa.[27]Hofland J, Herrera-Martínez AD, Zandee WT, et al. Management of carcinoid syndrome: a systematic review and meta-analysis. Endocr Relat Cancer. 2019 Mar;26(3):R145-56.
https://www.doi.org/10.1530/ERC-18-0495
http://www.ncbi.nlm.nih.gov/pubmed/30608900?tool=bestpractice.com
Second-line treatment for management of symptoms involves the use of radionuclide-targeted therapy or hepatic transarterial embolisation and possibly chemotherapy. However, chemotherapy for midgut carcinoids lacks efficacy, with response rates of <25%.[27]Hofland J, Herrera-Martínez AD, Zandee WT, et al. Management of carcinoid syndrome: a systematic review and meta-analysis. Endocr Relat Cancer. 2019 Mar;26(3):R145-56.
https://www.doi.org/10.1530/ERC-18-0495
http://www.ncbi.nlm.nih.gov/pubmed/30608900?tool=bestpractice.com
Somatostatin analogues
Somatostatin analogues are suitable for all patients with carcinoid syndrome. Octreotide was the first developed somatostatin analogue.[28]Lamberts SW, van der Lely AJ, de Herder WW, et al. Octreotide. N Engl J Med. 1996 Jan 25;334(4):246-54.
http://www.ncbi.nlm.nih.gov/pubmed/8532003?tool=bestpractice.com
This can be administered as a subcutaneous or intravenous injection. However, due to the short half-life, a 3-times-daily regimen was previously required to maintain steady levels.[29]Shah T, Caplin M. Endocrine tumours of the gastrointestinal tract. Biotherapy for metastatic endocrine tumours. Best Pract Res Clin Gastroenterol. 2005 Aug;19(4):617-36.
http://www.ncbi.nlm.nih.gov/pubmed/16183531?tool=bestpractice.com
As a result, long-acting preparations have been developed that last for 28 days. Both long-acting preparations have similar comparable binding profiles and bind with a high affinity to somatostatin receptor types 1 and 4.[30]O'Toole D, Ducreux M, Bommelaer G, et al. Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer. 2000 Feb 15;88(4):770-6.
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/%28SICI%291097-0142%2820000215%2988%3A4%3C770%3A%3AAID-CNCR6%3E3.0.CO%3B2-0
http://www.ncbi.nlm.nih.gov/pubmed/10679645?tool=bestpractice.com
[31]Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004 Jun;15(6):966-73.
http://annonc.oxfordjournals.org/cgi/content/full/15/6/966
http://www.ncbi.nlm.nih.gov/pubmed/15151956?tool=bestpractice.com
[32]Plockinger U, Wiedenmann B. Neuroendocrine tumors. Biotherapy. Best Pract Res Clin Endocrinol Metab. 2007 Mar;21(1):145-62.
http://www.ncbi.nlm.nih.gov/pubmed/17382270?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Octreotide planar image showing uptake in the liver from tumourFrom the collection of Dr R. Srirajaskanthan and Dr M. Caplin; used with permission [Citation ends].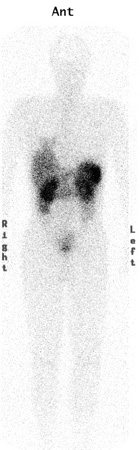
A randomised placebo-controlled prospective study demonstrated that somatostatin analogues inhibited tumour growth and delayed time to progression in patients with metastatic midgut tumours.[33]Rinke A, Mueller HH, Schade-Brittinger C, et al; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009 Oct 1;27(28):4656-63.
http://jco.ascopubs.org/content/27/28/4656.long
http://www.ncbi.nlm.nih.gov/pubmed/19704057?tool=bestpractice.com
A further study including small bowel neuroendocrine tumours (NETs) has confirmed these findings.[34]Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014 Jul 17;371(3):224-33.
http://www.nejm.org/doi/full/10.1056/NEJMoa1316158#t=article
http://www.ncbi.nlm.nih.gov/pubmed/25014687?tool=bestpractice.com
Somatostatin analogues also inhibit release of hormones from the pancreas. Steatorrhoea can occur with sustained use of these agents and is best treated with a pancreatic enzyme supplement.
Telotristat ethyl
Telotristat ethyl (formulated as telotristat etiprate, the hippurate salt of telotristat ethyl) is an orally delivered small molecule that acts by inhibiting the enzyme tryptophan hydroxylase (TPH), the rate-limiting enzyme responsible for serotonin production. Telotristat ethyl was specifically designed to be absorbed into the bloodstream without crossing the blood-brain barrier so as not to affect brain serotonin levels. Results from a placebo-controlled, phase 2 study in patients with metastatic carcinoid syndrome who were refractory to currently available therapy indicated that telotristat ethyl was well tolerated and demonstrated positive response in key measures, including reduction in bowel movements and in patient-reported adequate relief of carcinoid symptoms.[35]Kulke MH, O'Dorisio T, Phan A, et al. Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Relat Cancer. 2014 Oct;21(5):705-14.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4295770
http://www.ncbi.nlm.nih.gov/pubmed/25012985?tool=bestpractice.com
[36]Pavel M, Hörsch D, Caplin M, et al. Telotristat etiprate for carcinoid syndrome: a single-arm, multicenter trial. J Clin Endocrinol Metab. 2015 Apr;100(4):1511-9.
http://www.ncbi.nlm.nih.gov/pubmed/25636046?tool=bestpractice.com
The results of one study comparing telotristat ethyl with placebo in patients with high bowel frequency on the background of carcinoid syndrome has demonstrated a reduction in bowel frequency of patients receiving telotristat ethyl versus placebo.[37]Kulke MH, Hörsch D, Caplin ME, et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol. 2017 Jan;35(1):14-23.
http://ascopubs.org/doi/full/10.1200/JCO.2016.69.2780
http://www.ncbi.nlm.nih.gov/pubmed/27918724?tool=bestpractice.com
The European Medicines Agency (EMA) has recommended approval of telotristat ethyl in patients with carcinoid syndrome-related diarrhoea in combination with a somatostatin analogue. The US Food and Drug Administration (FDA) has approved the drug for use in patients with carcinoid syndrome-related diarrhoea who are not adequately controlled on a somatostatin analogue.
Hepatic transarterial embolisation
Liver metastases are often the cause of carcinoid syndrome and, therefore, if symptoms progress despite optimal medical management with biotherapy, there may be a role for hepatic embolisation.[38]Toumpanakis C, Meyer T, Caplin ME. Cytotoxic treatment including embolization/chemoembolization for neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007 Mar;21(1):131-44.
http://www.ncbi.nlm.nih.gov/pubmed/17382269?tool=bestpractice.com
The technique involves identifying the arterial blood supply to the hepatic metastases. If bilobar disease is present, then usually only 1 lobe is embolised at a time. Symptomatic improvement occurs in 40% to 80% and a biochemical response in 7% to 75% of cases.[39]Eriksson BK, Larsson EG, Skogseid BM, et al. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998 Dec 1;83(11):2293-301.
http://www.ncbi.nlm.nih.gov/pubmed/9840528?tool=bestpractice.com
[40]Ruszniewski P, Rougier P, Roche A, et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors: a prospective phase II study in 24 patients. Cancer. 1993 Apr 15;71(8):2624-30.
http://onlinelibrary.wiley.com/doi/10.1002/1097-0142(19930415)71:8%3C2624::AID-CNCR2820710830%3E3.0.CO;2-B/epdf
http://www.ncbi.nlm.nih.gov/pubmed/8384072?tool=bestpractice.com
The duration of response may last for 6 to 8 months, sometimes much longer.[38]Toumpanakis C, Meyer T, Caplin ME. Cytotoxic treatment including embolization/chemoembolization for neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007 Mar;21(1):131-44.
http://www.ncbi.nlm.nih.gov/pubmed/17382269?tool=bestpractice.com
Embolisation can be repeated, although its effectiveness diminishes with repeated episodes. Patients should be given intravenous fluids and allopurinol (to prevent tumour lysis syndrome) prior to the procedure and hospitalised for 24 to 72 hours post-procedure. Appropriate intravenous antibiotics should be commenced prior to procedure. Patients also require octreotide infusion to prevent carcinoid crisis, commenced at least 2 hours prior to surgery and given until 48 hours after surgery.
Selective internal radiotherapy treatment (SIRT) is a method of combining embolisation and radionuclide therapy to liver metastases. The radiotherapy is delivered by resin microsphere labelled with Yttrium-90. The 90-Y labelled microspheres are selectively delivered to the metastases via infusion through a catheter in the hepatic artery.[41]King J, Quinn R, Glenn DM, Janssen J, Tong D, Liaw W, Morris DL. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008 Sep 1;113(5):921-9.
http://onlinelibrary.wiley.com/doi/10.1002/cncr.23685/full
http://www.ncbi.nlm.nih.gov/pubmed/18618495?tool=bestpractice.com
Radionuclide therapies
The high-intensity expression of somatostatin receptors in NETs is demonstrated by a positive octreoscan, which involves the use of indium 111-In-diethylenetriaminepentaacetic acid (DTPA)-octreotide or Ga-68 DOTATATE or DOTATOC positive emission tomography (PET) imaging. Somatostatin receptors are present in the majority of carcinoid tumours. This facilitates the use of radionuclide therapies composed of a radiolabelled ligand attached to a somatostatin analogue, producing localised radionuclide activity. Radiolabelled somatostatin analogues can be given for inoperable or metastasised NETs. Patients to be considered for this therapy need to have a positive octreoscan or Ga-68 PET imaging. Patients need to be able to provide self-care, because for 24 hours they will be alone in a radioactive room.
Inclusion criteria are:
Good tumour uptake on 111-In-DTPA-octreotide scintigrams (tumour uptake > liver uptake)
Haemoglobin >80 g/L (8 g/dL)
WBC count >3.5 x 10⁹/L
Platelet count >80 x 10⁹/L
Creatinine clearance >40 mL/minute
The commonly used radionuclide therapies are yttrium Y 90 DOTA-octreotate and lutetium Lu 177 DOTA-octreotate.[42]Kwekkeboom DJ, Teunissen JJ, Bakker WH, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005 Apr 20;23(12):2754-62.
http://jco.ascopubs.org/cgi/content/full/23/12/2754
http://www.ncbi.nlm.nih.gov/pubmed/15837990?tool=bestpractice.com
[43]Van Essen M, Krenning EP, de Jong M, et al. Peptide receptor radionuclide therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007;46(6):723-34.
http://www.ncbi.nlm.nih.gov/pubmed/17653893?tool=bestpractice.com
Symptomatic improvement has been reported in 60% to 80% of cases. Partial tumour response of >50% tumour load is seen in 9% to 33% of patients, while disease stabilisation is reported in approximately two-thirds of cases. Both agents described have similar efficacy. Results of the first phase 3 randomised controlled trial examining Lu-177-DOTA-octreotate therapy in small bowel NET with carcinoid syndrome demonstrated an improved progression-free survival and possible overall survival benefit of Lu-177-DOTA-octreotate compared with octreotide (depot formulation).[44]Ruszniewski P. 177-Lu-Dotatate significantly improves progression-free survival in patients with midgut neuroendocrinetumours: results of the phase I NETTER-1 trial. Abstract 6LBA. European Cancer Congress; 2015. The use of radionucleotide therapies should be limited to specialist centres.
Other treatments
Generally, chemotherapy has disappointing results in management of symptoms in patients with carcinoid syndrome. The commonly used regimens depend in part on the histology and site of the primary tumour. For bronchial tumours, etoposide and cisplatin can be used as first-line chemotherapy, while other centres recommend capecitabine and temozolomide.[38]Toumpanakis C, Meyer T, Caplin ME. Cytotoxic treatment including embolization/chemoembolization for neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007 Mar;21(1):131-44.
http://www.ncbi.nlm.nih.gov/pubmed/17382269?tool=bestpractice.com
[45]Al-Toubah T, Morse B, Strosberg J. Capecitabine and temozolomide in advanced lung neuroendocrine neoplasms. Oncologist. 2020 Jan;25(1):e48-e52.
https://www.doi.org/10.1634/theoncologist.2019-0361
http://www.ncbi.nlm.nih.gov/pubmed/31455747?tool=bestpractice.com
Temozolomide is an oral alkylating agent used for the treatment of NETs as monotherapy. Results from one study showed a 14% radiological response, 53% had stable disease, and the overall median time to progression was 7 months.[46]Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007 May 15;13(10):2986-91.
http://clincancerres.aacrjournals.org/cgi/content/full/13/10/2986
http://www.ncbi.nlm.nih.gov/pubmed/17505000?tool=bestpractice.com
For midgut carcinoid tumours, the best clinical response rate identified was in a study using doxorubicin and streptozocin, where a 40% response rate was reported.[47]Frame J, Kelsen D, Kemeny N, et al. A phase II trial of streptozotocin and adriamycin in advanced APUD tumors. Am J Clin Oncol. 1988 Aug;11(4):490-5.
http://www.ncbi.nlm.nih.gov/pubmed/2841843?tool=bestpractice.com
Other studies report response rates of usually <25% following a number of different chemotherapy regimens for well-differentiated midgut NETs.[27]Hofland J, Herrera-Martínez AD, Zandee WT, et al. Management of carcinoid syndrome: a systematic review and meta-analysis. Endocr Relat Cancer. 2019 Mar;26(3):R145-56.
https://www.doi.org/10.1530/ERC-18-0495
http://www.ncbi.nlm.nih.gov/pubmed/30608900?tool=bestpractice.com
The response rate for poorly differentiated NETs with etoposide and cisplatin has been shown to be between 40% and 67%.[48]Moertel CG, Kvols LK, O'Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991 Jul 15;68(2):227-32.
http://www.ncbi.nlm.nih.gov/pubmed/1712661?tool=bestpractice.com
This response rate does not necessarily correlate with improvement in carcinoid symptoms and often is related to tumour-related symptoms such as weight loss and tiredness.[49]Bajetta E, Rimassa L, Carnaghi C, et al. 5-Fluorouracil, dacarbazine, and epirubicin in the treatment of patients with neuroendocrine tumors. Cancer. 1998 Jul 15;83(2):372-8.
https://acsjournals.onlinelibrary.wiley.com/doi/full/10.1002/(SICI)1097-0142(19980715)83:2%3C372::AID-CNCR23%3E3.0.CO;2-P
http://www.ncbi.nlm.nih.gov/pubmed/9669822?tool=bestpractice.com
Protocols, dosing and combination of agents tend to vary, and chemotherapy should only be used in specialist centres.
Everolimus and sunitinib are licensed for use in pancreatic NETs.[27]Hofland J, Herrera-Martínez AD, Zandee WT, et al. Management of carcinoid syndrome: a systematic review and meta-analysis. Endocr Relat Cancer. 2019 Mar;26(3):R145-56.
https://www.doi.org/10.1530/ERC-18-0495
http://www.ncbi.nlm.nih.gov/pubmed/30608900?tool=bestpractice.com
[50]Halfdanarson TR, Strosberg JR, Tang L, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of pancreatic neuroendocrine tumors. Pancreas. 2020 Aug;49(7):863-81.
https://www.doi.org/10.1097/MPA.0000000000001597
http://www.ncbi.nlm.nih.gov/pubmed/32675783?tool=bestpractice.com
Around 1% to 2% of pancreatic NETs cause carcinoid syndrome. Therefore, there is very limited evidence for improvement of carcinoid syndrome specifically with either of these agents.[50]Halfdanarson TR, Strosberg JR, Tang L, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of pancreatic neuroendocrine tumors. Pancreas. 2020 Aug;49(7):863-81.
https://www.doi.org/10.1097/MPA.0000000000001597
http://www.ncbi.nlm.nih.gov/pubmed/32675783?tool=bestpractice.com
However, there is excellent evidence demonstrating delayed time to progression using these agents compared with placebo.[51]Walter MA, Nesti C, Spanjol M, et al. Treatment for gastrointestinal and pancreatic neuroendocrine tumours: a network meta-analysis. Cochrane Database Syst Rev. 2021 Nov 25;11:CD013700.
https://www.doi.org/10.1002/14651858.CD013700.pub2
http://www.ncbi.nlm.nih.gov/pubmed/34822169?tool=bestpractice.com
Everolimus is a protein kinase inhibitor of mTOR (mammalian target of rapamycin) that has demonstrated prolonged progression-free survival in the RADIANT-3 study.[52]Yao JC, Shah MH, Ito T, et al; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011 Feb 10;364(6):514-23.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4208619
http://www.ncbi.nlm.nih.gov/pubmed/21306238?tool=bestpractice.com
Results from one RADIANT-4 study have demonstrated a progression-free survival benefit in patients with gastrointestinal and bronchial NETs.[53]Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016 Mar 5;387(10022):968-77.
http://www.ncbi.nlm.nih.gov/pubmed/26703889?tool=bestpractice.com
Sunitinib inhibits cellular signalling by targeting multiple tyrosine kinase receptors including: platelet-derived growth factor receptor (PDGF-R), vascular endothelial growth factor receptor (VEGF-R), KIT, and RET.[54]Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011 Feb 10;364(6):501-13.
http://www.nejm.org/doi/full/10.1056/NEJMoa1003825#t=article
http://www.ncbi.nlm.nih.gov/pubmed/21306237?tool=bestpractice.com
Interferon alfa has previously been used in patients with carcinoid syndrome who are intolerant of somatostatin analogues. Symptomatic response is observed in approximately 40% of patients.[29]Shah T, Caplin M. Endocrine tumours of the gastrointestinal tract. Biotherapy for metastatic endocrine tumours. Best Pract Res Clin Gastroenterol. 2005 Aug;19(4):617-36.
http://www.ncbi.nlm.nih.gov/pubmed/16183531?tool=bestpractice.com
[32]Plockinger U, Wiedenmann B. Neuroendocrine tumors. Biotherapy. Best Pract Res Clin Endocrinol Metab. 2007 Mar;21(1):145-62.
http://www.ncbi.nlm.nih.gov/pubmed/17382270?tool=bestpractice.com
[55]Oberg K, Eriksson B. The role of interferons in the management of carcinoid tumours. Br J Haematol. 1991 Oct;79(suppl 1):74-7.
http://www.ncbi.nlm.nih.gov/pubmed/1834159?tool=bestpractice.com
The effects of interferon alfa are mediated via the interferon type 1 receptors. In addition to its symptom control effects, it has anti-proliferative and anti-angiogenic actions.[56]Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors: the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003 Jul 15;21(14):2689-96.
http://www.ncbi.nlm.nih.gov/pubmed/12860945?tool=bestpractice.com
The most commonly used interferon is interferon alfa-2a or interferon alfa-2b. The biochemical response varies between 15% and 45%. The pegylated interferon preparations are also available for use as weekly injections.[57]Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999 Mar 18;340(11):858-68.
http://www.ncbi.nlm.nih.gov/pubmed/10080850?tool=bestpractice.com
Cyproheptadine has been used only on rare occasions where somatostatin analogues and interferon alfa are unable to control diarrhoea. It appears to be effective in the management of diarrhoea associated with malignant carcinoid syndrome. However, its biochemical and antitumoural effects are minimal.[58]Moertel CG, Kvols LK, Rubin J. A study of cyproheptadine in the treatment of metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer. 1991 Jan 1;67(1):33-6.
http://www.ncbi.nlm.nih.gov/pubmed/1985720?tool=bestpractice.com
