Treatment goals include the following: improvement to WHO functional class I or II, improvement in the 6-minute walk distance (6MWD) to 380-440 metres, normal or near normal right ventricular (RV) size and function on echocardiography, a peak oxygen uptake of >15 mL/kg/minute during cardiopulmonary exercise testing, normal B-type natriuretic peptide (BNP) levels, and normalisation of RV function as assessed by haemodynamics obtained by right heart catheterisation (right atrial pressure <8 mmHg and cardiac index >2.5 L/minute/m²).[55]McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol. 2013 Dec 24;62(25 Suppl):D73-81.
https://www.jacc.org/doi/10.1016/j.jacc.2013.10.034
http://www.ncbi.nlm.nih.gov/pubmed/24355644?tool=bestpractice.com
Due to the complexities that these patients pose, it is strongly recommended that they are referred to a centre with expertise in the treatment of pulmonary arterial hypertension (PAH). Treatment options available include general supportive therapy, pulmonary artery hypertension-specific therapy (calcium-channel blockers, prostanoids, endothelin receptor antagonists, phosphodiesterase-5 [PDE5] inhibitors, a selective prostacyclin IP receptor agonist [selexipag], a soluble guanylate cyclase stimulator [riociguat], or an activin signalling inhibitor [sotatercept]), and lung transplantation as appropriate.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[35]Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest. 2019 Mar;155(3):565-86.[Erratum in: Chest. 2021 Jan;159(1):457.]
http://www.ncbi.nlm.nih.gov/pubmed/30660783?tool=bestpractice.com
[56]Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019 Jan;53(1):1801889.
https://erj.ersjournals.com/content/53/1/1801889
http://www.ncbi.nlm.nih.gov/pubmed/30545971?tool=bestpractice.com
Supportive therapy
General supportive therapy is indicated for most patients.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[35]Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest. 2019 Mar;155(3):565-86.[Erratum in: Chest. 2021 Jan;159(1):457.]
http://www.ncbi.nlm.nih.gov/pubmed/30660783?tool=bestpractice.com
[56]Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019 Jan;53(1):1801889.
https://erj.ersjournals.com/content/53/1/1801889
http://www.ncbi.nlm.nih.gov/pubmed/30545971?tool=bestpractice.com
Supervised exercise training: recommended for those on PAH medical therapy who are in a stable clinical condition.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[57]Rochester CL, Alison JA, Carlin B, et al. Pulmonary rehabilitation for adults with chronic respiratory disease: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2023 Aug 15;208(4):e7-26.
https://www.atsjournals.org/doi/10.1164/rccm.202306-1066ST
http://www.ncbi.nlm.nih.gov/pubmed/37581410?tool=bestpractice.com
[58]Grünig E, Eichstaedt C, Barberà JA, et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J. 2019 Feb;53(2).
https://erj.ersjournals.com/content/53/2/1800332
http://www.ncbi.nlm.nih.gov/pubmed/30578391?tool=bestpractice.com
[59]Morris NR, Kermeen FD, Jones AW, et al. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst Rev. 2023 Mar 22;3(3):CD011285.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011285.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/36947725?tool=bestpractice.com
[  ]
What are the effects of exercise‐based rehabilitation programs for adults with pulmonary hypertension?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4304/fullShow me the answer Heavy physical exertion and isometric exercise should be avoided.[60]McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. J Am Coll Cardiol. 2009 Apr 28;53(17):1573-619.
https://www.jacc.org/doi/10.1016/j.jacc.2009.01.004
http://www.ncbi.nlm.nih.gov/pubmed/19389575?tool=bestpractice.com
There is a lack of evidence for a direct impact of exercise training on survival and outcome in pulmonary hypertension. However, there are studies showing a beneficial effect on prognostically important parameters. The European Respiratory Society has identified a strong need to establish specialised rehabilitation programmes for patients with PAH to enhance access to this treatment intervention, which appears to be effective, cost-efficient and safe.[58]Grünig E, Eichstaedt C, Barberà JA, et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J. 2019 Feb;53(2).
https://erj.ersjournals.com/content/53/2/1800332
http://www.ncbi.nlm.nih.gov/pubmed/30578391?tool=bestpractice.com
]
What are the effects of exercise‐based rehabilitation programs for adults with pulmonary hypertension?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.4304/fullShow me the answer Heavy physical exertion and isometric exercise should be avoided.[60]McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. J Am Coll Cardiol. 2009 Apr 28;53(17):1573-619.
https://www.jacc.org/doi/10.1016/j.jacc.2009.01.004
http://www.ncbi.nlm.nih.gov/pubmed/19389575?tool=bestpractice.com
There is a lack of evidence for a direct impact of exercise training on survival and outcome in pulmonary hypertension. However, there are studies showing a beneficial effect on prognostically important parameters. The European Respiratory Society has identified a strong need to establish specialised rehabilitation programmes for patients with PAH to enhance access to this treatment intervention, which appears to be effective, cost-efficient and safe.[58]Grünig E, Eichstaedt C, Barberà JA, et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J. 2019 Feb;53(2).
https://erj.ersjournals.com/content/53/2/1800332
http://www.ncbi.nlm.nih.gov/pubmed/30578391?tool=bestpractice.com
Psychosocial support: should be considered, including advanced care planning with referral to specialist palliative care services at the right time.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Vaccinations: patients should be offered influenza, Streptococcus pneumoniae, and coronavirus disease 2019 (COVID-19) vaccinations at a minimum.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Diuretics: recommended in patients with fluid retention and signs of right ventricular failure.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[61]Badesch DB, Abman SH, Simonneau G, et al. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007 Jun;131(6):1917-28.
http://www.ncbi.nlm.nih.gov/pubmed/17565025?tool=bestpractice.com
[62]Alam S, Palevsky HI. Standard therapies for pulmonary arterial hypertension. Clin Chest Med. 2007 Mar;28(1):91-115.
http://www.ncbi.nlm.nih.gov/pubmed/17338930?tool=bestpractice.com
Supplemental oxygen: given in hypoxaemia.[4]Humbert M, Sitbon O, Guignabert C, et al. Treatment of pulmonary arterial hypertension: recent progress and a look to the future. Lancet Respir Med. 2023 Sep;11(9):804-19.
https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(23)00264-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37591298?tool=bestpractice.com
Supplemental iron: correction of iron status in case of iron deficiency.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Anticoagulation: not generally recommended in patients with PAH but should be considered on a case-by-case basis.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Pregnancy: patients with IPAH should avoid pregnancy, and women of child-bearing age should be counselled about the risks associated with becoming pregnant.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[35]Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest. 2019 Mar;155(3):565-86.[Erratum in: Chest. 2021 Jan;159(1):457.]
http://www.ncbi.nlm.nih.gov/pubmed/30660783?tool=bestpractice.com
Those who present during pregnancy, or who become pregnant, should be treated by a multidisciplinary team experienced in managing pulmonary hypertension in pregnancy. Some of the drugs used to treat IPAH may cause fetal harm and may not be recommended in pregnancy; consult your local drug information source for more information.
Acute vasoreactivity testing
In some patients, vasoconstriction largely predominates over vascular remodeling phenomena. In this situation, calcium channel blockers can provide real clinical benefit.[4]Humbert M, Sitbon O, Guignabert C, et al. Treatment of pulmonary arterial hypertension: recent progress and a look to the future. Lancet Respir Med. 2023 Sep;11(9):804-19.
https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(23)00264-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37591298?tool=bestpractice.com
These patients are identified by acute vasoreactivity testing (with inhaled nitric oxide, inhaled iloprost, or intravenous epoprostenol), which should generally be performed in all IPAH patients. Patients who are responsive should be treated with optimally tolerated doses of calcium-channel blockers. It is estimated that <10% of patients with IPAH are responders.[63]Naranjo M, Rosenzweig EB, Hemnes AR, et al. Frequency of acute vasodilator response (AVR) in incident and prevalent patients with pulmonary arterial hypertension: Results from the pulmonary vascular disease phenomics study. Pulm Circ. 2023 Jul;13(3):e12281.
https://onlinelibrary.wiley.com/doi/10.1002/pul2.12281
http://www.ncbi.nlm.nih.gov/pubmed/37614830?tool=bestpractice.com
Vasoreactivity testing is contraindicated in patients with WHO functional class IV symptoms and low cardiac index. Vasoreactivity testing is generally not recommended for pulmonary hypertension that is caused by underlying diseases.
Positive response:
A positive response is defined as a fall in mean pulmonary artery pressure by ≥10 mmHg to reach an absolute value ≤40 mmHg, with an increased or unchanged cardiac output.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[60]McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. J Am Coll Cardiol. 2009 Apr 28;53(17):1573-619.
https://www.jacc.org/doi/10.1016/j.jacc.2009.01.004
http://www.ncbi.nlm.nih.gov/pubmed/19389575?tool=bestpractice.com
About 12% of IPAH patients may have a positive response and should be started on calcium-channel blocker therapy.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[47]Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005 Jun 14;111(23):3105-11.
https://www.ahajournals.org/doi/full/10.1161/circulationaha.104.488486
http://www.ncbi.nlm.nih.gov/pubmed/15939821?tool=bestpractice.com
A satisfactory response to calcium-channel blocker therapy is defined as being in WHO functional class I or II with near-normal haemodynamics after several months of therapy.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[47]Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005 Jun 14;111(23):3105-11.
https://www.ahajournals.org/doi/full/10.1161/circulationaha.104.488486
http://www.ncbi.nlm.nih.gov/pubmed/15939821?tool=bestpractice.com
Close monitoring is mandatory, and patients should have a full re-assessment at 3-6 months.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Patients with a sustained satisfactory response should continue calcium-channel blocker therapy, with further re-assessment every 6-12 months.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Patients without a sustained satisfactory response require additional therapy and should be treated the same as those with a negative response to acute vasoreactivity testing. Continuation of calcium-channel blocker (combined with additional therapy) may need to be considered in some patients because of clinical deterioration with calcium-channel blocker withdrawal attempts.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Calcium-channel blockers predominantly used in PAH are nifedipine, diltiazem, felodipine, and amlodipine.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Choice of drug is based on baseline heart rate (HR): if HR <100 beats/minute - nifedipine, felodipine, amlodipine; if HR >100 beats/minute - diltiazem.
Non-responders to vasoreactivity testing and patients without sustained response to calcium-channel blockers, or those in whom calcium-channel blockers are contraindicated, should be started on another PAH-specific therapy. All currently approved drugs target the vasoconstriction-vasodilation balance of endothelial dysfunction and vascular smooth muscle cell proliferation observed in PAH.[4]Humbert M, Sitbon O, Guignabert C, et al. Treatment of pulmonary arterial hypertension: recent progress and a look to the future. Lancet Respir Med. 2023 Sep;11(9):804-19.
https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(23)00264-3/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/37591298?tool=bestpractice.com
Targeted treatment options include the following.
Prostanoids: prostanoids have been shown to improve the distance walked in 6 minutes, functional class, and haemodynamics, and to avoid clinical worsening.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[64]Barnes H, Yeoh HL, Fothergill T, et al. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2019 May 1;5(5):CD012785.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012785.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/31042010?tool=bestpractice.com
[  ]
What are the benefits and harms of prostacyclin for people with pulmonary arterial hypertension?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2737/fullShow me the answer Epoprostenol is administered via continuous intravenous infusion (requiring an infusion pump and a permanent central venous catheter, with associated risks of central venous catheter bloodstream infections). In one prospective, randomised, multi-centre trial, continuous intravenous infusion of epoprostenol improved symptoms, haemodynamics, and survival in patients with severe IPAH.[65]Barst RJ, Rubin LJ, Long WA, et al; Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996 Feb 1;334(5):296-301.
https://www.nejm.org/doi/full/10.1056/NEJM199602013340504
http://www.ncbi.nlm.nih.gov/pubmed/8532025?tool=bestpractice.com
Other prostanoids include treprostinil, which can be administered subcutaneously or intravenously.
[
]
What are the benefits and harms of prostacyclin for people with pulmonary arterial hypertension?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2737/fullShow me the answer Epoprostenol is administered via continuous intravenous infusion (requiring an infusion pump and a permanent central venous catheter, with associated risks of central venous catheter bloodstream infections). In one prospective, randomised, multi-centre trial, continuous intravenous infusion of epoprostenol improved symptoms, haemodynamics, and survival in patients with severe IPAH.[65]Barst RJ, Rubin LJ, Long WA, et al; Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996 Feb 1;334(5):296-301.
https://www.nejm.org/doi/full/10.1056/NEJM199602013340504
http://www.ncbi.nlm.nih.gov/pubmed/8532025?tool=bestpractice.com
Other prostanoids include treprostinil, which can be administered subcutaneously or intravenously.
[  ]
What are the benefits and harms of prostacyclin for people with pulmonary arterial hypertension?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2737/fullShow me the answer Subcutaneous administration of treprostinil avoids the risks associated with chronic indwelling central venous catheters, and in one double-blind, placebo-controlled multi-centre trial was shown to improve exercise capacity, symptoms, and haemodynamics in patients with PAH.[66]Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002 Mar 15;165(6):800-4.
https://www.atsjournals.org/doi/10.1164/ajrccm.165.6.2106079
http://www.ncbi.nlm.nih.gov/pubmed/11897647?tool=bestpractice.com
]
What are the benefits and harms of prostacyclin for people with pulmonary arterial hypertension?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2737/fullShow me the answer Subcutaneous administration of treprostinil avoids the risks associated with chronic indwelling central venous catheters, and in one double-blind, placebo-controlled multi-centre trial was shown to improve exercise capacity, symptoms, and haemodynamics in patients with PAH.[66]Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002 Mar 15;165(6):800-4.
https://www.atsjournals.org/doi/10.1164/ajrccm.165.6.2106079
http://www.ncbi.nlm.nih.gov/pubmed/11897647?tool=bestpractice.com
Endothelin receptor antagonists: endothelin-1 binds to endothelin A and B receptors and is a potent vasoconstrictor and smooth-muscle mitogen whose system is activated in IPAH.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
One Cochrane review found that for people with WHO functional class II and III PAH, endothelin receptor antagonists probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, and improve haemodynamics.[67]Liu C, Chen J, Gao Y, et al. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2021 Mar 26;3(3):CD004434.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004434.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/33765691?tool=bestpractice.com
[  ]
For people with pulmonary arterial hypertension, what are the effects of endothelin receptor antagonists (ERAs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3647/fullShow me the answer Bosentan, an oral antagonist of both endothelin A and B receptors, has shown improvements in the 6MWD, functional class, haemodynamics, and time to clinical worsening. Liver function tests should be checked every month and haematocrit every 3 months. Bosentan is teratogenic in animals and may lessen the effectiveness of hormonal contraception. It may cause testicular atrophy and male infertility.[60]McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. J Am Coll Cardiol. 2009 Apr 28;53(17):1573-619.
https://www.jacc.org/doi/10.1016/j.jacc.2009.01.004
http://www.ncbi.nlm.nih.gov/pubmed/19389575?tool=bestpractice.com
Ambrisentan, a selective endothelin A receptor antagonist, improves exercise capacity, symptoms, and haemodynamics and carries a low risk of liver toxicity.[68]Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-blind, Placebo-controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation. 2008 Jun 10;117(23):3010-9.
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.107.742510
http://www.ncbi.nlm.nih.gov/pubmed/18506008?tool=bestpractice.com
[69]Galiè N, Badesch D, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005 Aug 2;46(3):529-35.
https://www.jacc.org/doi/10.1016/j.jacc.2005.04.050
http://www.ncbi.nlm.nih.gov/pubmed/16053970?tool=bestpractice.com
Macitentan is a novel dual endothelin receptor antagonist found to delay a composite endpoint of morbidity and mortality (driven by worsening PAH symptoms) in one long-term event-driven placebo-controlled clinical trial.[70]Pulido T, Adzerikho I, Channick RN, et al; SERAPHIN Investigators. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013 Aug 29;369(9):809-18.
https://www.nejm.org/doi/full/10.1056/NEJMoa1213917
http://www.ncbi.nlm.nih.gov/pubmed/23984728?tool=bestpractice.com
]
For people with pulmonary arterial hypertension, what are the effects of endothelin receptor antagonists (ERAs)?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.3647/fullShow me the answer Bosentan, an oral antagonist of both endothelin A and B receptors, has shown improvements in the 6MWD, functional class, haemodynamics, and time to clinical worsening. Liver function tests should be checked every month and haematocrit every 3 months. Bosentan is teratogenic in animals and may lessen the effectiveness of hormonal contraception. It may cause testicular atrophy and male infertility.[60]McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. J Am Coll Cardiol. 2009 Apr 28;53(17):1573-619.
https://www.jacc.org/doi/10.1016/j.jacc.2009.01.004
http://www.ncbi.nlm.nih.gov/pubmed/19389575?tool=bestpractice.com
Ambrisentan, a selective endothelin A receptor antagonist, improves exercise capacity, symptoms, and haemodynamics and carries a low risk of liver toxicity.[68]Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-blind, Placebo-controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation. 2008 Jun 10;117(23):3010-9.
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.107.742510
http://www.ncbi.nlm.nih.gov/pubmed/18506008?tool=bestpractice.com
[69]Galiè N, Badesch D, Oudiz R, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005 Aug 2;46(3):529-35.
https://www.jacc.org/doi/10.1016/j.jacc.2005.04.050
http://www.ncbi.nlm.nih.gov/pubmed/16053970?tool=bestpractice.com
Macitentan is a novel dual endothelin receptor antagonist found to delay a composite endpoint of morbidity and mortality (driven by worsening PAH symptoms) in one long-term event-driven placebo-controlled clinical trial.[70]Pulido T, Adzerikho I, Channick RN, et al; SERAPHIN Investigators. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013 Aug 29;369(9):809-18.
https://www.nejm.org/doi/full/10.1056/NEJMoa1213917
http://www.ncbi.nlm.nih.gov/pubmed/23984728?tool=bestpractice.com
PDE5 inhibitors: these agents augment the pulmonary vascular response to nitric oxide (NO).[71]Ruopp NF, Cockrill BA. Diagnosis and treatment of pulmonary arterial hypertension: a review. JAMA. 2022 Apr 12;327(14):1379-91. [Erratum in: JAMA. 2022 Sep 6;328(9):892.]
http://www.ncbi.nlm.nih.gov/pubmed/35412560?tool=bestpractice.com
Tadalafil has been shown to improve the 6MWD and to be associated with less clinical worsening and improved quality of life.[72]Galiè N, Brundage BH, Ghofrani HA, et al; Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009 Jun 9;119(22):2894-903.
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.108.839274
http://www.ncbi.nlm.nih.gov/pubmed/19470885?tool=bestpractice.com
[  ]
What are the effects of phosphodiesterase 5 inhibitors (PDE5is) for people with pulmonary artery hypertension?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2454/fullShow me the answer Sildenafil has been shown to improve exercise capacity, functional class, and haemodynamics in patients with PAH, and may reduce clinical worsening.[73]Galiè N, Ghofrani HA, Torbicki A, et al; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005 Nov 17;353(20):2148-57.
https://www.nejm.org/doi/full/10.1056/NEJMoa050010
http://www.ncbi.nlm.nih.gov/pubmed/16291984?tool=bestpractice.com
[74]Barnes H, Brown Z, Burns A, et al. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst Rev. 2019 Jan 31;1(1):CD012621.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012621.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/30701543?tool=bestpractice.com
]
What are the effects of phosphodiesterase 5 inhibitors (PDE5is) for people with pulmonary artery hypertension?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.2454/fullShow me the answer Sildenafil has been shown to improve exercise capacity, functional class, and haemodynamics in patients with PAH, and may reduce clinical worsening.[73]Galiè N, Ghofrani HA, Torbicki A, et al; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005 Nov 17;353(20):2148-57.
https://www.nejm.org/doi/full/10.1056/NEJMoa050010
http://www.ncbi.nlm.nih.gov/pubmed/16291984?tool=bestpractice.com
[74]Barnes H, Brown Z, Burns A, et al. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst Rev. 2019 Jan 31;1(1):CD012621.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012621.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/30701543?tool=bestpractice.com
Soluble guanylate cyclase stimulators: also augment the NO pathway, but they do so by directly stimulating the enzyme that synthesises cyclic guanosine monophosphate (cGMP), the second messenger and downstream effector of NO. Riociguat, the only drug available in this class to date, improved 6MWD, pulmonary vascular resistance, N-terminal pro-B-type natriuretic peptide, WHO functional class, and time to clinical worsening in one 12-week, double-blind, multi-centre randomised trial.[75]Ghofrani HA, Galiè N, Grimminger F, et al; PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013 Jul 25;369(4):330-40.
https://www.nejm.org/doi/full/10.1056/NEJMoa1209655
http://www.ncbi.nlm.nih.gov/pubmed/23883378?tool=bestpractice.com
Selective prostacyclin IP receptor agonists: selexipag, the only drug available in this class, reduced the risk of a composite of death or complications related to PAH in both pre-treated patients and patients naive to therapy. Lower rates of disease progression and hospitalisation accounted for the majority of the risk reduction.[76]Sitbon O, Channick R, Chin KM, et al; GRIPHON Investigators. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015 Dec 24;373(26):2522-33.
https://www.nejm.org/doi/full/10.1056/NEJMoa1503184
http://www.ncbi.nlm.nih.gov/pubmed/26699168?tool=bestpractice.com
Activin signalling inhibitor: sotatercept is a recombinant fusion protein composed of the extracellular domain of the human activin receptor type IIA linked to the FC domain of human IgG1, which acts as a ligand trap by binding activins and GDFs, thus restoring the balance between pro-proliferative and anti-proliferative bone morphogenetic protein (BMP) pathways.[77]Martin de Miguel I, Cruz-Utrilla A, Oliver E, et al. Novel molecular mechanisms involved in the medical treatment of pulmonary arterial hypertension. Int J Mol Sci. 2023 Feb 19;24(4):4147
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9965798
http://www.ncbi.nlm.nih.gov/pubmed/36835558?tool=bestpractice.com
Sotatercept is approved in the US for the treatment of adults with PAH to increase exercise capacity, improve WHO functional class, and reduce the risk of clinical worsening events. It is also approved in Europe for this indication. In one phase 2 trial, treatment with sotatercept resulted in a reduction in pulmonary vascular resistance in patients receiving background therapy for PAH, compared with placebo.[78]Humbert M, McLaughlin V, Gibbs JSR, et al; PULSAR Trial Investigators. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021 Apr 1;384(13):1204-15.
https://www.nejm.org/doi/10.1056/NEJMoa2024277
http://www.ncbi.nlm.nih.gov/pubmed/33789009?tool=bestpractice.com
In the multi-centre, double-blind, phase 3 STELLAR trial, in patients with PAH (WHO functional class II or III) on stable background therapy, adding sotatercept significantly improved exercise capacity (as assessed by the 6-minute walk test), compared with placebo.[79]Hoeper MM, Badesch DB, Ghofrani HA, et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023 Apr 20;388(16):1478-90.
https://www.nejm.org/doi/10.1056/NEJMoa2213558
http://www.ncbi.nlm.nih.gov/pubmed/36877098?tool=bestpractice.com
Risk stratification as a basis for treatment decisions
For treatment decisions, a rational approach should be used to classify patients into risk groups based on the combination of several clinical features, including haemodynamics, WHO functional class, 6MWD, and BNP or N-terminal pro-BNP (NT-proBNP).[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[56]Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019 Jan;53(1):1801889.
https://erj.ersjournals.com/content/53/1/1801889
http://www.ncbi.nlm.nih.gov/pubmed/30545971?tool=bestpractice.com
In general, the goal of therapy is to shift patients to, or maintain patients in, a low-risk category. Significant clinical judgement and experience is needed to make these decisions.
For patients without cardiopulmonary comorbidities, the European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines recommend a three-strata model for risk assessment at the time of diagnosis, using categories of low, intermediate, and high risk of mortality at 1 year to assess survival and guide management.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[54]Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017 Aug;50(2):1700740.
https://erj.ersjournals.com/content/50/2/1700740
http://www.ncbi.nlm.nih.gov/pubmed/28775047?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Comprehensive risk assessment in pulmonary arterial hypertension (three-strata model)European Heart Journal. 2022 Oct 7;43(38):3618-731; used with permission [Citation ends].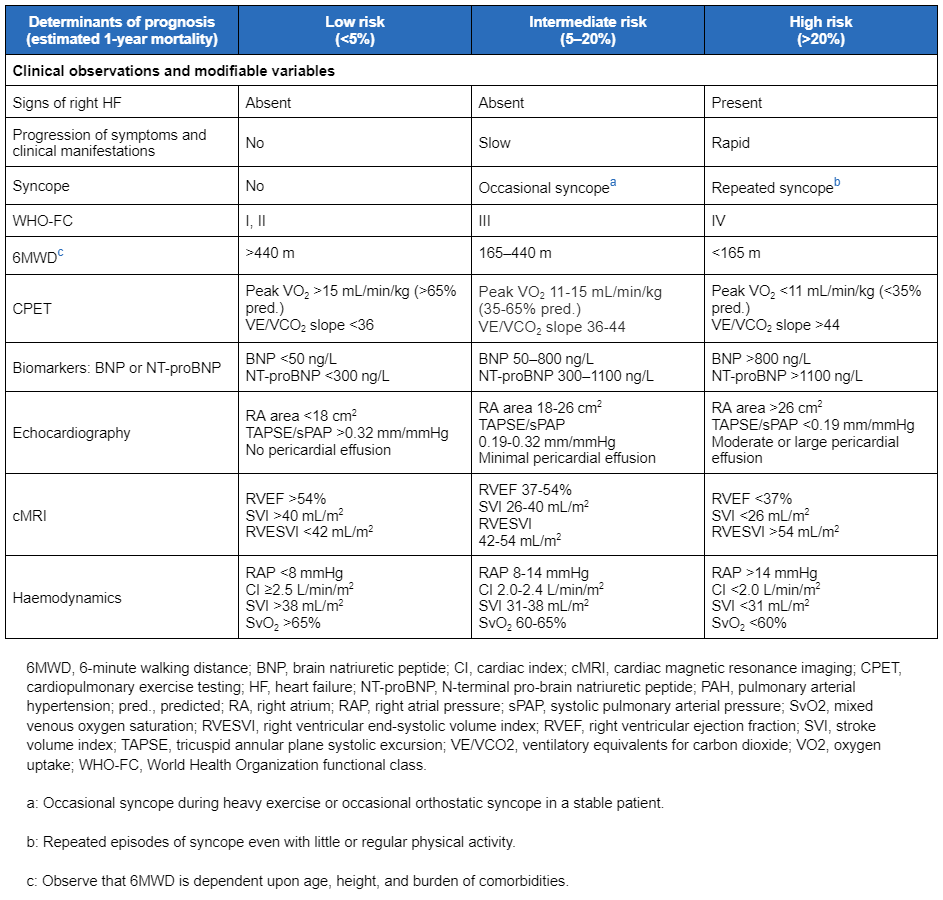 Patients initially assessed as at low or intermediate risk of mortality who are unresponsive to acute vasoreactivity testing, or who cannot take calcium-channel blockers, should preferably be started on combination therapy with an endothelin receptor antagonist plus a PDE5 inhibitor.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
ESC/ERS guidelines recommend initial oral combination therapy with tadalafil plus either ambrisentan or macitentan.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Combinations of other endothelin receptor antagonists and PDE5 inhibitors may also be considered.
Patients initially assessed as at low or intermediate risk of mortality who are unresponsive to acute vasoreactivity testing, or who cannot take calcium-channel blockers, should preferably be started on combination therapy with an endothelin receptor antagonist plus a PDE5 inhibitor.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
ESC/ERS guidelines recommend initial oral combination therapy with tadalafil plus either ambrisentan or macitentan.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Combinations of other endothelin receptor antagonists and PDE5 inhibitors may also be considered.
One multi-centre, randomised, double-blind, phase 3 study showed a lower risk of clinical failure with upfront combination therapy with ambrisentan plus tadalafil compared with each monotherapy alone.[80]Galiè N, Barbera JA, Frost AE, et al: AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015 Aug 27;373(9):834-44.
https://www.nejm.org/doi/full/10.1056/NEJMoa1413687
http://www.ncbi.nlm.nih.gov/pubmed/26308684?tool=bestpractice.com
In this study, upfront combination therapy with ambrisentan plus tadalafil delayed clinical worsening in PAH, particularly hospitalisations. Another multi-centre, randomised, double-blind, phase 3b study demonstrated substantial improvements in haemodynamics and exercise capacity with initial combination therapy (macitentan and tadalafil) and that there was no benefit of triple therapy (macitentan, tadalafil, and selexipag) compared with the double therapy.[81]Chin KM, Sitbon O, Doelberg M, et al. Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol. 2021 Oct 5;78(14):1393-403.
https://www.jacc.org/doi/10.1016/j.jacc.2021.07.057
http://www.ncbi.nlm.nih.gov/pubmed/34593120?tool=bestpractice.com
Patients initially assessed as being at high risk of mortality who are unresponsive to acute vasoreactivity testing, or who cannot take calcium-channel blockers, should be considered for combination therapy with an endothelin receptor antagonist plus a PDE5 inhibitor plus a parenteral prostanoid (intravenous epoprostenol or intravenous/subcutaneous treprostinil).[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Upfront triple therapy is associated with clinical and haemodynamic improvement and reduces right ventricular remodelling.[82]Haarman MG, Lévy M, Roofthooft MTR, et al. Upfront triple combination therapy in severe paediatric pulmonary arterial hypertension. Eur Respir J. 2021 Jan;57(1):2001120.
https://erj.ersjournals.com/content/57/1/2001120
http://www.ncbi.nlm.nih.gov/pubmed/32855224?tool=bestpractice.com
[83]Sitbon O, Jaïs X, Savale L, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J. 2014 Jun;43(6):1691-7.
https://erj.ersjournals.com/content/43/6/1691
http://www.ncbi.nlm.nih.gov/pubmed/24627535?tool=bestpractice.com
[84]D'Alto M, Badagliacca R, Argiento P, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest. 2020 Feb;157(2):376-83.
http://www.ncbi.nlm.nih.gov/pubmed/31563498?tool=bestpractice.com
During monitoring and follow-up after initial therapy (every 3-6 months according to patient needs), the ESC/ERS guidelines recommend using a four-strata model, which uses categories of low, intermediate-low, intermediate-high, and high risk to guide treatment decisions.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Variables used to calculate the simplified four-strata risk-assessment toolEuropean Heart Journal. 2022 Oct 7;43(38):3618-731; used with permission [Citation ends].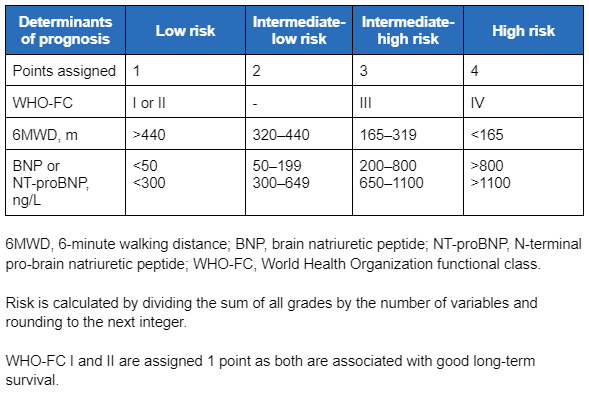 Patients assessed to be at low risk should continue their initial therapy.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Switching from intravenous or subcutaneous therapy to oral therapy in patients who achieve a low risk status can be considered on a case-by-case basis.
Patients assessed to be at low risk should continue their initial therapy.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Switching from intravenous or subcutaneous therapy to oral therapy in patients who achieve a low risk status can be considered on a case-by-case basis.
Addition of selexipag to the initial combination of an endothelin receptor antagonist plus a PDE5 inhibitor should be considered for patients assessed to be at intermediate-low risk. Alternatively, switching from the PDE5 inhibitor to riociguat may also be considered.[85]Hoeper MM, Al-Hiti H, Benza RL, et al; REPLACE investigators. Switching to riociguat versus maintenance therapy with phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): a multicentre, open-label, randomised controlled trial. Lancet Respir Med. 2021 Jun;9(6):573-84.
http://www.ncbi.nlm.nih.gov/pubmed/33773120?tool=bestpractice.com
A PDE5 inhibitor and riociguat should not be used in combination, because this leads to a higher risk of systemic hypotension.[86]Wardle AJ, Seager MJ, Wardle R, et al. Guanylate cyclase stimulators for pulmonary hypertension. Cochrane Database Syst Rev. 2016 Aug 2;2016(8):CD011205.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011205.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27482837?tool=bestpractice.com
Addition of a prostanoid should be considered for patients assessed to be at intermediate-high or high risk while on an initial combination of an endothelin receptor antagonist plus a PDE5 inhibitor.[86]Wardle AJ, Seager MJ, Wardle R, et al. Guanylate cyclase stimulators for pulmonary hypertension. Cochrane Database Syst Rev. 2016 Aug 2;2016(8):CD011205.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011205.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/27482837?tool=bestpractice.com
Referral for lung transplantation evaluation should also be considered.
Patients with cardiopulmonary comorbidities
Evidence for treatment of patients with cardiopulmonary comorbidities is lacking. The ESC/ERS guidelines recommend that initial monotherapy with an endothelin receptor antagonist or a PDE5 inhibitor may be considered, with additional treatment options considered on an individual basis for those at intermediate or high risk of death at follow-up.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Treatment-resistant patients
Patients refractory to all medical therapy should be evaluated for lung transplantation.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
The availability of effective medical therapy has reduced the need for lung transplantation. However, transplantation remains an important option for patients in whom medical therapy has failed and who remain in WHO functional class III or IV. Referral for lung transplant evaluation is recommended when patients present with an inadequate response to optimised combination therapy and have an intermediate-high or high risk of death.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Double lung transplantation is most commonly performed. Heart-lung transplantation should be considered in patients with additional cardiac conditions.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
When to list a patient for lung transplantation is a difficult decision, further complicated by the unpredictable waiting list and the shortage of donor organs.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
Balloon atrial septostomy (BAS) produces right-to-left shunting that decompresses the right atrium and right ventricle, increases systemic cardiac output, and decreases systemic arterial oxygen saturation. The latter is offset by the increase in output leading to an increase in systemic oxygen transport.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
BAS is used as a palliative option or bridge to lung transplantation in patients who are deteriorating despite maximal medical therapy; as it is a complex and high-risk procedure it is rarely performed and only considered in experienced centres.[3]Humbert M, Kovacs G, Hoeper MM, et al; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618-731. [Erratum in: Eur Heart J. 2023 Feb 23:ehad005.]
https://academic.oup.com/eurheartj/article/43/38/3618/6673929
http://www.ncbi.nlm.nih.gov/pubmed/36017548?tool=bestpractice.com
[56]Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019 Jan;53(1):1801889.
https://erj.ersjournals.com/content/53/1/1801889
http://www.ncbi.nlm.nih.gov/pubmed/30545971?tool=bestpractice.com
 ]
Heavy physical exertion and isometric exercise should be avoided.[60] There is a lack of evidence for a direct impact of exercise training on survival and outcome in pulmonary hypertension. However, there are studies showing a beneficial effect on prognostically important parameters. The European Respiratory Society has identified a strong need to establish specialised rehabilitation programmes for patients with PAH to enhance access to this treatment intervention, which appears to be effective, cost-efficient and safe.[58]
]
Heavy physical exertion and isometric exercise should be avoided.[60] There is a lack of evidence for a direct impact of exercise training on survival and outcome in pulmonary hypertension. However, there are studies showing a beneficial effect on prognostically important parameters. The European Respiratory Society has identified a strong need to establish specialised rehabilitation programmes for patients with PAH to enhance access to this treatment intervention, which appears to be effective, cost-efficient and safe.[58] ]
Epoprostenol is administered via continuous intravenous infusion (requiring an infusion pump and a permanent central venous catheter, with associated risks of central venous catheter bloodstream infections). In one prospective, randomised, multi-centre trial, continuous intravenous infusion of epoprostenol improved symptoms, haemodynamics, and survival in patients with severe IPAH.[65] Other prostanoids include treprostinil, which can be administered subcutaneously or intravenously.
[
]
Epoprostenol is administered via continuous intravenous infusion (requiring an infusion pump and a permanent central venous catheter, with associated risks of central venous catheter bloodstream infections). In one prospective, randomised, multi-centre trial, continuous intravenous infusion of epoprostenol improved symptoms, haemodynamics, and survival in patients with severe IPAH.[65] Other prostanoids include treprostinil, which can be administered subcutaneously or intravenously.
[  ]
Subcutaneous administration of treprostinil avoids the risks associated with chronic indwelling central venous catheters, and in one double-blind, placebo-controlled multi-centre trial was shown to improve exercise capacity, symptoms, and haemodynamics in patients with PAH.[66]
]
Subcutaneous administration of treprostinil avoids the risks associated with chronic indwelling central venous catheters, and in one double-blind, placebo-controlled multi-centre trial was shown to improve exercise capacity, symptoms, and haemodynamics in patients with PAH.[66] ]
Bosentan, an oral antagonist of both endothelin A and B receptors, has shown improvements in the 6MWD, functional class, haemodynamics, and time to clinical worsening. Liver function tests should be checked every month and haematocrit every 3 months. Bosentan is teratogenic in animals and may lessen the effectiveness of hormonal contraception. It may cause testicular atrophy and male infertility.[60] Ambrisentan, a selective endothelin A receptor antagonist, improves exercise capacity, symptoms, and haemodynamics and carries a low risk of liver toxicity.[68][69] Macitentan is a novel dual endothelin receptor antagonist found to delay a composite endpoint of morbidity and mortality (driven by worsening PAH symptoms) in one long-term event-driven placebo-controlled clinical trial.[70]
]
Bosentan, an oral antagonist of both endothelin A and B receptors, has shown improvements in the 6MWD, functional class, haemodynamics, and time to clinical worsening. Liver function tests should be checked every month and haematocrit every 3 months. Bosentan is teratogenic in animals and may lessen the effectiveness of hormonal contraception. It may cause testicular atrophy and male infertility.[60] Ambrisentan, a selective endothelin A receptor antagonist, improves exercise capacity, symptoms, and haemodynamics and carries a low risk of liver toxicity.[68][69] Macitentan is a novel dual endothelin receptor antagonist found to delay a composite endpoint of morbidity and mortality (driven by worsening PAH symptoms) in one long-term event-driven placebo-controlled clinical trial.[70] ]
Sildenafil has been shown to improve exercise capacity, functional class, and haemodynamics in patients with PAH, and may reduce clinical worsening.[73][74]
]
Sildenafil has been shown to improve exercise capacity, functional class, and haemodynamics in patients with PAH, and may reduce clinical worsening.[73][74] Patients initially assessed as at low or intermediate risk of mortality who are unresponsive to acute vasoreactivity testing, or who cannot take calcium-channel blockers, should preferably be started on combination therapy with an endothelin receptor antagonist plus a PDE5 inhibitor.[3] ESC/ERS guidelines recommend initial oral combination therapy with tadalafil plus either ambrisentan or macitentan.[3] Combinations of other endothelin receptor antagonists and PDE5 inhibitors may also be considered.
Patients initially assessed as at low or intermediate risk of mortality who are unresponsive to acute vasoreactivity testing, or who cannot take calcium-channel blockers, should preferably be started on combination therapy with an endothelin receptor antagonist plus a PDE5 inhibitor.[3] ESC/ERS guidelines recommend initial oral combination therapy with tadalafil plus either ambrisentan or macitentan.[3] Combinations of other endothelin receptor antagonists and PDE5 inhibitors may also be considered. Patients assessed to be at low risk should continue their initial therapy.[3] Switching from intravenous or subcutaneous therapy to oral therapy in patients who achieve a low risk status can be considered on a case-by-case basis.
Patients assessed to be at low risk should continue their initial therapy.[3] Switching from intravenous or subcutaneous therapy to oral therapy in patients who achieve a low risk status can be considered on a case-by-case basis.