Approach
Treatment usually consists of the following components:
Brief intervention, particularly in unhealthy alcohol use and mild alcohol-use disorder
Individual therapy
Psychosocial support
Medications to support reduced alcohol intake and abstinence
Ongoing support to help the patient maintain their goals regarding alcohol intake.
Patients may benefit from a combination of interventions, and the use of one should not preclude the use of another.[60][61]
Goals of treatment may include abstinence, reduction or moderation of alcohol use, or other elements of harm reduction, and should be agreed between patient and clinician. The patient’s legal obligations, and risk of harm to self or others from alcohol use, should also be discussed.[40]
The appropriate level of care for a patient is determined via six dimensions:
Withdrawal risk
Biomedical conditions or complications
Stability of co-occurring mood disorders and cognitive status
Readiness to change
Likelihood of return to use
Living environment and psychosocial support.
Patients who are medically unstable or do not meet their goals with outpatient management can be stepped up to a higher level of care. Lack of access to a higher level of care, however, should not prevent clinicians from offering medication treatment, support services, and harm reduction strategies.
[Figure caption and citation for the preceding image starts]: Treatment overviewCreated by BMJ Knowledge Centre [Citation ends].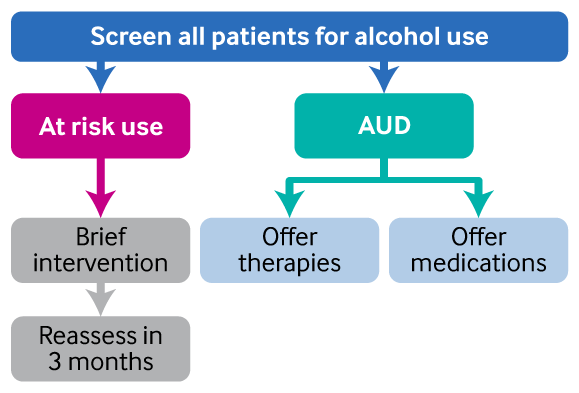
Management of withdrawal
One of the most life-threatening complications of alcohol-use disorder is alcohol withdrawal. As a result, telling patients who use alcohol daily to stop drinking abruptly could be dangerous. At the time of assessment, patients should be assessed with a structured instrument, such as the revised Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar), to determine the severity of the alcohol withdrawal syndrome (AWS).[62] Clinical Institute Withdrawal Assessment of Alcohol Scale, revised (CIWA- Ar) Opens in new window
For further details on inpatient management see Alcohol withdrawal.
Brief interventions
People with unhealthy alcohol use that do not meet alcohol-use disorder criteria can often be treated with a brief intervention. These interventions are typically 5-15 minutes long, follow a structured outline, provide education, and may or may not include follow-up treatment. In patients with at-risk drinking, these interventions reduce total alcohol consumed.[63][64] Most studies excluded patients with moderate to severe alcohol-use disorder, and evidence for the effectiveness of brief intervention without referral to treatment in this population as well as in those with multiple substance use disorders is lacking.
Brief interventions can consist of one or more sessions in the clinic or hospital setting (e.g., emergency department or inpatient unit), during which education and feedback about the patient's alcohol use and its consequences are provided in a supportive and empathic fashion.
[  ]
Brief interventions may be improved by follow-up; for instance, one multi-site randomised controlled trial (RCT) in the trauma setting showed improvement in several drinking measures at up to 12 months for patients who received an individualised follow-up phone call in addition to brief motivational interviewing, compared with those who just received the interview.[65] A formal means of assessing the effectiveness of the plan and follow-up visits with the clinician should be part of the plan.[66] Though brief interventions are easy to administer, they go beyond simply telling patients to reduce their alcohol use: in studies of their effectiveness, clinicians were trained to provide structured feedback based on an individual’s risk and desire to change their behaviour. One commonly used structure is the FRAMES method: Feedback, Responsibility, Advice, Menu of options, Empathic style, Self-efficacy.
]
Brief interventions may be improved by follow-up; for instance, one multi-site randomised controlled trial (RCT) in the trauma setting showed improvement in several drinking measures at up to 12 months for patients who received an individualised follow-up phone call in addition to brief motivational interviewing, compared with those who just received the interview.[65] A formal means of assessing the effectiveness of the plan and follow-up visits with the clinician should be part of the plan.[66] Though brief interventions are easy to administer, they go beyond simply telling patients to reduce their alcohol use: in studies of their effectiveness, clinicians were trained to provide structured feedback based on an individual’s risk and desire to change their behaviour. One commonly used structure is the FRAMES method: Feedback, Responsibility, Advice, Menu of options, Empathic style, Self-efficacy.
[Figure caption and citation for the preceding image starts]: FRAMES method for brief interventionAdapted by Best Practice topic authors from: Bien TH et al. Addiction. 1993 Mar;88(3):315-35 [Citation ends].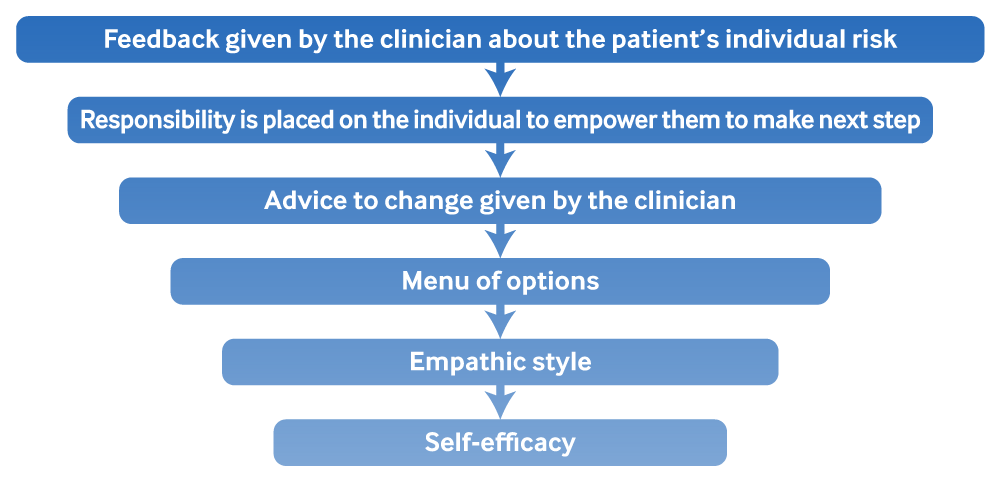
Motivational interviewing
Motivational interviewing, a technique that assists the patient in identifying their own goals, is an effective, patient-centred way of engaging patients in treatment and reducing substance use.[67] Motivational interviewing requires clinicians to avoid confronting, labelling, or directing the patient, but rather to elicit the patient’s ambivalence and support his/her motivation to change through open-ended questions, affirmations, reflective statements, and summarising.
Psychosocial interventions
Non-pharmacological approaches can be useful for many patients with alcohol-use disorder, as they provide strategies to help patients avoid returning to alcohol use, enhance self-efficacy, and reduce the impact of stressors that can precipitate return to use. Interventions may be individual or group-based. There is no strong evidence for any one approach, and therefore the patient’s preference should guide treatment choice.[68]
Outpatient and intensive outpatient addiction treatment programmes typically include treatment sessions scheduled from once- or twice-weekly to several times a week, with treatment extending over several weeks to months (or longer). The interventions provided may include: cognitive behavioural therapy (to assist patients in coping with thoughts of return to alcohol use and negative cognitions), counselling strategies (return-to-use prevention, coping with stressors, forming beneficial relationships), and referral of patients to self-help groups, such as Alcoholics Anonymous (AA).[12][69][70] In a meta-analysis of 6 studies, the addition of manualised cognitive behavioural therapy did not improve return-to-use rates when added to naltrexone therapy.[71]
AA, founded in 1939, is the most common programme. Its primary goal is to help individuals maintain total abstinence from alcohol and other addictive substances.
Alcoholics Anonymous
Opens in new window
[  ]
[Evidence A] Self-help programmes such as AA can provide valuable additional support for many patients and their families. Patients indicating benefit from support group attendance should be encouraged to attend the group meetings over an extended timeframe; some patients may benefit from attending indefinitely. In one large RCT, AA improved drinking outcomes, largely through improvements in adaptive social network changes and social self-efficacy.[72] Each AA group is independent, so patients should be encouraged to try several if one is not a good fit. For those who have negative experiences or views of AA, it is important that clinicians are aware of alternatives, such as Smart Recovery groups.
]
[Evidence A] Self-help programmes such as AA can provide valuable additional support for many patients and their families. Patients indicating benefit from support group attendance should be encouraged to attend the group meetings over an extended timeframe; some patients may benefit from attending indefinitely. In one large RCT, AA improved drinking outcomes, largely through improvements in adaptive social network changes and social self-efficacy.[72] Each AA group is independent, so patients should be encouraged to try several if one is not a good fit. For those who have negative experiences or views of AA, it is important that clinicians are aware of alternatives, such as Smart Recovery groups.
The following table summarises psychological interventions in the outpatient setting.
[Figure caption and citation for the preceding image starts]: Non-pharmacological approaches can be useful for many patients with alcohol-use disorder, as they provide strategies to help patients avoid returning to alcohol use, enhance self-efficacy, and reduce the impact of stressors that can precipitate return to useTable created by authors of the Best Practice topic [Citation ends].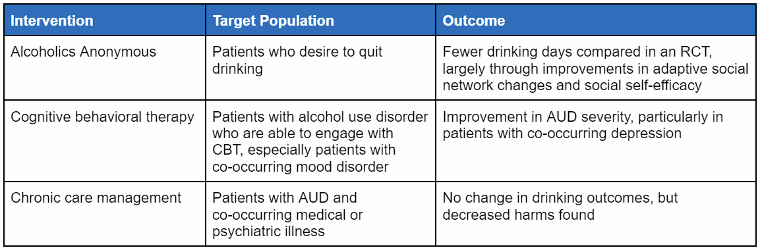
Pharmacological treatment
Pharmacological treatment can help support abstinence, reduce alcohol consumed, and reduce the number of heavy drinking days, all of which are likely to reduce harm from alcohol use.[60] Nonetheless, medications for alcohol-use disorder remain under-utilised because of lack of clinician and patient familiarity, and perhaps a tendency to discount outcomes other than abstinence as worthwhile clinical goals. Three medications - naltrexone, acamprosate, and disulfiram - are approved by the US Food and Drug Administration (FDA) for the treatment of alcohol-use disorder. Naltrexone, acamprosate, and disulfiram have been studied in patients with moderate to severe alcohol-use disorder, but may be offered to patients with mild alcohol-use disorder on a case-by-case basis.[40] The use of medications does not preclude other psychosocial support; and psychosocial support does not preclude the use of medications. Indeed, the greatest efficacy may be seen in patients using a combination of medication and psychosocial treatment.[60] Medications should be continued for 6 months after abstinence is achieved, if that is the patient's goal.[60] Further treatment is determined through shared decision-making.
Naltrexone is an opioid receptor antagonist that decreases alcohol use by attenuating the rewarding and reinforcing effects of alcohol. Specifically, naltrexone blocks stimulation of the opioid receptor by endogenous opioids and decreases dopamine release in the ventral tegmental area.[73] It is particularly useful in patients with a family history of alcohol-use disorder and in those with significant alcohol cravings.[12][40][55] Oral naltrexone can be started in patients who are still drinking, and it is usually well tolerated. Naltrexone can precipitate opioid withdrawal in those with opioids in their system. It is not started in patients until they have been opioid-free for several days (7-10 days) and is contraindicated in patients who require opioid agonists for pain or opioid use disorder. Naltrexone can cause stomach discomfort and a mild increase in liver function tests, especially in the oral formulation. As such, the oral formulation is not recommended in patients with alanine aminotransferase (ALT) measurements greater than 5 times the normal limit. Intramuscular naltrexone appears to be safe except in acute liver failure or decompensated cirrhosis. As it does not undergo first pass metabolism in the liver, serum concentration is more predictable and it is considered safer.[74][75][76] Treatment with naltrexone reduces heavy drinking days, improves abstinence, and reduces the risk of returning to any or heavy drinking at 3 and 12 months post-treatment, compared with placebo.[77] [
 ]
In a meta-analysis of 122 studies, the number needed to treat (NNT) to prevent return to drinking for oral naltrexone was 20, and the NNT to reduce heavy drinking was 12.[78] The intramuscular formulation of naltrexone may be more effective in patients with difficulty taking daily medications.[79] Naltrexone can be used as a monotherapy or in combination with gabapentin, topiramate, or acamprosate.
]
In a meta-analysis of 122 studies, the number needed to treat (NNT) to prevent return to drinking for oral naltrexone was 20, and the NNT to reduce heavy drinking was 12.[78] The intramuscular formulation of naltrexone may be more effective in patients with difficulty taking daily medications.[79] Naltrexone can be used as a monotherapy or in combination with gabapentin, topiramate, or acamprosate. Gabapentin reduces heavy drinking days and return to drinking in patients with prominent alcohol withdrawal symptoms. The exact mechanism of gabapentin is not known, but it appears to inhibit the release of excitatory neurotransmitters. In an RCT of 96 individuals with alcohol-use disorder, gabapentin resulted in a statistically significant decrease in heavy drinking days (NNT 5) and preventing return to use (NNT 6) for patients with high baseline alcohol withdrawal symptoms.[80] As a result, in patients with symptoms of alcohol withdrawal, gabapentin is emerging as an effective treatment, either as monotherapy or in conjunction with naltrexone.[40][81] The main side effects of gabapentin are dizziness, drowsiness, and nausea. Patients should be counselled that abrupt cessation of gabapentin can lower the seizure threshold.
Topiramate is an anticonvulsant that has been shown in RCTs to decrease craving and withdrawal symptoms and significantly improve physical and psychosocial wellbeing of patients with alcohol-use disorder, compared with placebo (NNT 3 to reduce heavy drinking, NNT 7 to achieve abstinence).[82][83][84][85][86] It decreases percentage of heavy drinking days by >23% and increases abstinent days by almost 3 days.[87] Importantly, efficacy was established in subjects who were drinking at the time of starting the medication.[88] One study has shown that only individuals who are homozygous for the gene that codes for the kainate receptor protein had a reduction in heavy drinking days with topiramate treatment.[89] The benefits of topiramate should be weighed against the number needed to harm given its high side effect profile, including blurred vision, paraesthesias, poor memory, and loss of coordination.
Acamprosate normalises glutamate and gamma-aminobutyric acid neurotransmitter systems in the central nervous system. It is thought that these actions reduce ongoing symptoms associated with alcohol abstinence (e.g., anxiety, insomnia) and cravings. Pill burden may reduce its effectiveness. It is primarily metabolised by the kidneys, and a dose reduction may be required in patients with renal impairment (its use is contraindicated if creatinine clearance is <30 mL/minute).[40] Acamprosate increases the rate and duration of abstinence, compared with placebo.[90][91] Acamprosate is safe to use in patients who are actively drinking, but evidence for its use is from trials with people who have already stopped drinking. The NNT is 12 to prevent return to any drinking.[78] Naltrexone and acamprosate are equally efficacious.[78] [
 ]
]
Disulfiram blocks the catabolic pathway of alcohol by inhibiting aldehyde dehydrogenase, thereby increasing acetaldehyde levels following alcohol ingestion. The disulfiram-alcohol reaction can produce a number of somatic effects: vasomotor symptoms (flushing), cardiovascular symptoms (tachycardia, hypotension), digestive symptoms (nausea, vomiting, diarrhoea), headache, respiratory depression, and malaise. These symptoms are generally transient, but serious reactions may occur that require urgent medical treatment. Disulfiram is prescribed for those intending to abstain from alcohol use, acting through negative reinforcement to promote abstinence. Trials to support disulfiram use are limited. One meta-analysis showed that disulfiram was more effective than placebo, acamprosate, or naltrexone in open-label RCTs, but not in blinded RCTs.[92] Blinded studies are difficult to conduct, because the effectiveness of disulfiram depends on the patient's anticipation of somatic effects. Disulfiram therapy was more effective when supervised.[92]
The following table summarises the medications, their mechanism of action, and the number needed to treat to reduce heavy drinking and to achieve abstinence.
[Figure caption and citation for the preceding image starts]: Summary of medications, their mechanism of action, and the number needed to treat to reduce heavy drinking and to achieve abstinenceCreated by BMJ Knowledge Centre with information from: Anton RF et al. JAMA Intern Med. 2020;180(5):728-36; Johnson BA et al. Lancet. 2003;361(9370):1677-85; Winslow BT et al. Am Fam Physician. 2016;93(6):457-65; Lyon J. JAMA. 2017;317(22):2267-9; Murphy CE et al. Addiction. 2022; 117(2):271-81. [Citation ends].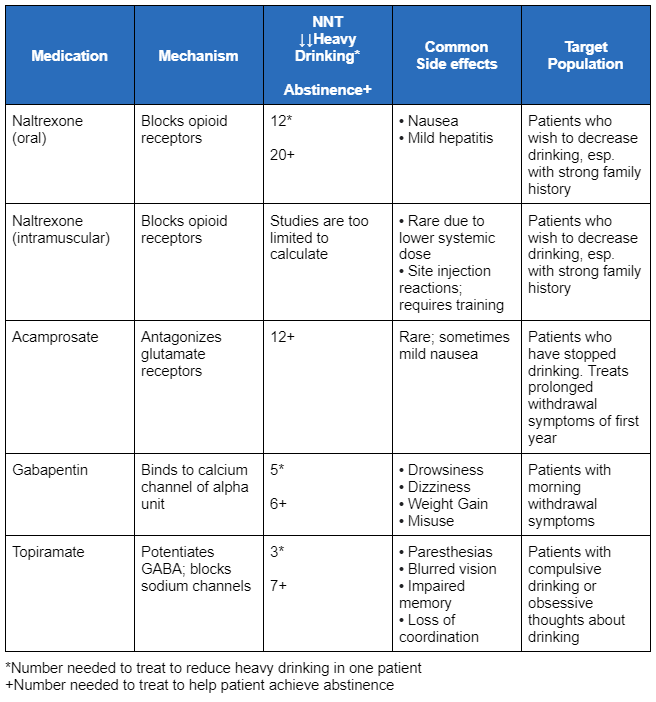
Management of medical comorbidities
Patients with unhealthy alcohol use often have co-existing high blood pressure, insomnia, weight gain, cognitive disturbances, gastrointestinal distress, and cardiac arrhythmias. It is important to emphasise that many of these conditions may worsen with alcohol use, even at low levels. Management of physical comorbidities and timely integrated care is important to improve the wellbeing of the patient.[93] Chronic care management reduces harms of alcohol-use disorder in patients with medical comorbidities.[94]
Pregnant women
Psychosocial interventions increase abstinence rates, compared with usual care or no intervention, in pregnant women who consume alcohol.[95]
There are no RCTs evaluating the effectiveness of pharmacological interventions to improve maternal, birth, and infant outcomes in pregnant women enrolled in alcohol treatment programmes.[96] Given the impact of alcohol on pregnancy, however, medications should be considered for women who are pregnant and are unable to stop drinking without pharmacological support.
Naltrexone is the best-studied in pregnant patients, although primarily in opioid use disorder, and is not associated with birth defects.[97] It is generally recommended to switch to the oral formulation at 36 weeks’ gestation to allow for effective pain management at birth.
Acamprosate is also an option if the benefits outweigh the risks, but its efficacy is unclear.[98]
Gabapentin may lead to withdrawal in neonates, especially in pregnant women with co-occurring alcohol-use disorder.
Topiramate is contraindicated in pregnancy. Topiramate is associated with a higher risk of congenital malformations and a high prevalence of babies being born small for gestational age.[99] It may also be associated with an approximately 2 to 3 times increased risk of intellectual disability, autistic spectrum disorders and attention deficit hyperactivity disorder.[100]
In some countries, topiramate is contraindicated in pregnancy and in women of child-bearing age unless the conditions of a pregnancy prevention programme are fulfilled to ensure that women of child-bearing potential: are using highly effective contraception; have a pregnancy test to exclude pregnancy before starting topiramate; and are aware of the risks associated with use of the drug.[99][101]
Disulfiram is not recommended in pregnant patients due to its gastrointestinal side effects and lack of safety data.
Adolescents
A meta-analysis of 16 studies demonstrated that psychosocial interventions are effective in reducing adolescent alcohol use, with individual-directed treatments possibly having a greater effect than family-based ones. Interventions with large effect sizes included brief motivational interviewing, cognitive behavioural therapy with 12 steps, cognitive behavioural therapy with aftercare, multidimensional family therapy, brief interventions with the adolescent, and brief interventions with the adolescent and a parent.[102] Group therapy-based treatments may be effective for adolescents, but safety should be considered and these modalities require further investigation.[103]
Naltrexone has been found to reduce cravings in adolescents in a few small trials. Acamprosate has likewise shown promise. Disulfiram can be useful for adolescents motivated to stop drinking, but has poor efficacy in those who do not desire abstinence and are not receiving it in directly observed therapy.[104]
Concurrent opioid use
Several step-by-step treatment recommendations have been developed to integrate psychosocial therapies with pharmacological therapies for alcohol and drug use disorders.[105][106] These procedures include patient education, personalised feedback, emotional support, medication monitoring, and motivational enhancement. National Institute on Alcohol Abuse and Alcoholism (NIAAA) Opens in new window
Choice of first-line pharmacological treatment options vary in people with concurrent opioid use. Naltrexone can precipitate opioid withdrawal in those with opioids in their system, so should be avoided. It is not started in patients until they have been opioid-free for several days (7-10 days) and is contraindicated in patients who require opioid agonists for pain or opioid use disorder.
Acamprosate and gabapentin may be used in patients with concurrent opioid use and alcohol-use disorder. Evidence indicates that patients with opioid use disorder and polydrug users are at risk for gabapentin misuse and clinicians should monitor usage closely.[107] Patients should also be counselled that abrupt cessation can lower seizure threshold.
Topiramate and disulfiram are second-line options.
Sedating substances or medications (e.g., benzodiazepines, barbiturates, opioids, muscle relaxants) may have similar signs and symptoms as alcohol-use disorder, or may be used together with alcohol. Use of these substances with alcohol may pose risk for overdose complications (e.g., respiratory depression).
Concurrent mental health diagnosis
Symptoms of alcohol-use disorder (including those of intoxication/withdrawal) may be confused with symptoms of other psychiatric disorders. Furthermore, other psychiatric disorders can co-occur with alcohol-use disorder. Observation over days to weeks to months may be necessary to clarify underlying psychiatric diagnoses. However, co-treatment generally has better outcomes than waiting for patients to stop drinking before making a diagnosis or starting psychiatric treatment; therefore, treatment should not be withheld until patients stop drinking.
Psychosocial treatments for alcohol-use disorder and naltrexone can be used first line. Gabapentin is emerging as an effective treatment option either as a monotherapy or in conjunction with naltrexone.[81] Topiramate can be considered second line and disulfiram third line.
A meta-analysis of 15 RCTs demonstrated reduced alcohol use and improved psychiatric outcomes when co-occurring depressive and anxiety disorders were treated concurrently with alcohol-use disorder.[108]
Treatment options depend on diagnosis and may include antidepressants. Selective serotonin-reuptake inhibitors (SSRIs) have demonstrated efficacy in alcohol-use disorder for patients with comorbid depression. Sertraline, when compared with placebo, decreases the proportion of days drinking during treatment and increases the number of patients who had continuous abstinence. Its efficacy was higher in those with more mild alcohol-use disorder.[109] Trials of fluoxetine for alcohol-use disorder achieved similar results, suggesting that fluoxetine may also be effective in mild alcohol-use disorder.[110] However, not all studies have supported the utility of SSRIs for treating alcohol-use disorder. A study of fluoxetine for people with alcohol-use disorder and co-occurring depression demonstrated no benefit of fluoxetine versus placebo on either depression or drinking.[111]
For more details on management of depression see Depression in adults.
Use of this content is subject to our disclaimer