Approach
General practitioners and primary care nurses are generally on the front line of care for patients with diabetes. As such, they have a key role in preventing and identifying active diabetic foot problems.
Diabetologists, specialist podiatrists, and other medical specialists are key in the evaluation and management of these patients, both in multidisciplinary diabetic foot clinics and when patients with diabetes are admitted for other acute medical conditions.
In the UK, the National Institute for Health and Care Excellence (NICE) recommends that any patient with an active diabetic foot problem is seen by the multidisciplinary foot care team within 1 working day.[9] If the problem is limb-threatening or life-threatening, refer the patient immediately to acute services and follow your local protocol to inform the multidisciplinary foot care service.[9]
A structured assessment of the risk of foot problems must be done:[9][36]
When diabetes is diagnosed and at least annually thereafter in all patients with diabetes (more frequently for those assessed to be at moderate or high risk of foot complications). See Screening for more details.
Whenever a patient with diabetes is admitted to hospital for any reason or if there is any change in their status during an admission
Whenever a patient with diabetes presents with a foot problem of any kind.
The main goals of the initial evaluation include:
Identifying the presence of any foot ulcers.
Assessing for any clinical symptoms or signs of infection, inflammation, or gangrene.
Assessing for the presence of sensory neuropathy, ideally using a 10-g monofilament, but if this is not available the Ipswich Touch Test is a suitable alternative (lightly touching the tips of the patient’s toes with the tip of your index finger for 1-2 seconds).[9][36]
Assessing for the presence of impaired vibration perception, using a 128-Hz tuning fork.[36]
Documenting pedal pulses.
Assessing for any signs of deformity.
This should be done even when there is no suspicion of diabetes-related foot disease.
Bear in mind that the absence of symptoms does not exclude foot disease. The patient may have asymptomatic neuropathy, peripheral arterial disease, pre-ulcerative signs, or even an ulcer.[9][36]
An active ulcer immediately requires a greater sense of urgency and should be classified according to the degree of tissue loss, the presence/degree of ischaemia, and the presence/degree of infection. The key factors associated with occurrence or recurrence include the presence of sensory neuropathy (loss of protective sensation); the presence of vascular disease; and/or a past history of an ulcer, Charcot's neuro-osteoarthropathy, or amputation. These three factors can easily be screened without complex equipment.[23]
History
Strong risk factors for diabetes-related foot disease include: sensory neuropathy, peripheral arterial disease, previous history of foot ulcer, previous history of major amputation, foot deformities, and end-stage renal disease.[9][12][14][21][22][23][24]
A diabetic foot ulcer is defined as a break in the skin that includes as a minimum the epidermis and part of the dermis and which occurs below/distal to the malleoli in a person with diabetes.[1]
Most patients who develop foot ulcers have at least some degree of sensory neuropathy. Sensory neuropathy blunts or obviates the nociceptive feedback that usually signals an injury sustained during ambulation or via a puncture wound. Lack of protective sensation due to sensory neuropathy is most often due to diabetes but can occasionally be due to other causes (e.g., alcohol abuse).
If an infection is present, it is common for patients to note the onset of foot pain in a previously insensate area. The presence of fever, chills, malaise, or anorexia is suggestive of an infection.[9]
Ask the patient whether they have had any history of micro- or macrovascular complications, claudication, or issues with glucose control.
Physical examination
Always examine the skin integrity of the foot and any muscular deformities in a well-lit room. When the feet are examined in a patient with diabetes, shoes, socks, bandages, and dressings should be removed, and both feet should be examined for evidence of:[9]
Neuropathy: NICE recommends using a 10-g monofilament; the International Working Group on the Diabetic Foot suggests the Ipswich Touch Test or a 128-Hz tuning fork can be used as an alternative[24]
Limb ischaemia (by palpation of pedal pulses, with absence of both pulses indicating peripheral arterial disease)
Ulceration
Callus formation
Infection and/or inflammation
Deformity
Gangrene
Charcot's neuro-osteoarthropathy
When examining the feet, pay special attention to weight-bearing areas, as the majority of non-healing foot ulcers and infections occur in these areas, resulting from repetitive trauma during ambulation on an insensate, sometimes structurally abnormal foot.
Patients with Charcot's neuro-osteoarthropathy leading to midfoot collapse may develop ulcers and infections in the midfoot that are associated with the structural abnormalities there.
Heel ulcers can develop due to pressure in non-ambulatory patients debilitated by other comorbidities or medical events (e.g., a history of stroke). They occur less frequently in ambulatory patients.
Whenever an ulcer is identified, document its size, depth, and position and classify it using a standardised system (for further details, see Diagnostic criteria). In the UK, NICE recommends use of the SINBAD system (Site, Ischaemia, Neuropathy, Bacterial Infection, Area, and Depth) to record the severity of the ulcer.[9] The result should be submitted to the NHS National Diabetes Foot Care Audit. NHS National Diabetes Foot Care Audit Opens in new window
Oedema and localised warmth of the foot, ankle, or calf is suggestive of infection. Erythema is suggestive of cellulitis, with or without a deep soft-tissue infection (i.e., abscess). Fluctuance is also suggestive of an abscess. Occasionally, infections are initiated by a puncture wound rather than ulceration from repetitive trauma. According to NICE and the IWGDF/Infectious Diseases Society of America (IDSA), a diabetic foot infection is defined by the presence of at least two of the following:[9][40]
Local swelling or induration
Erythema (>0.5 cm around the wound)
Local tenderness or pain
Local warmth
Purulent discharge
Classify and document the severity of any infection as mild, moderate or severe using the IWGDF/Infectious Diseases Society of America (IDSA) system.[40]
It is worth noting that because of the impaired immune response and abnormal arteriovenous shunting present in the neuropathic foot, clinical signs of infection in patients with diabetes may be more subtle than in non-diabetic patients.[Figure caption and citation for the preceding image starts]: Uninfected foot ulcer overlying the plantar aspect of the first metatarsophalangeal joint. Note the hyperkeratotic skin (callus) surrounding the wound edgeFrom the collection of Dr Neal R. Barshes; used with permission [Citation ends].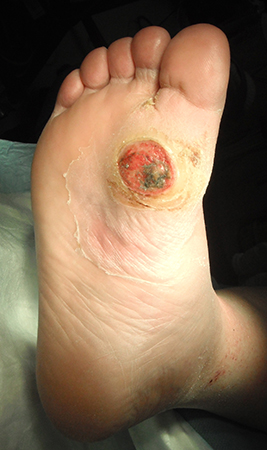 [Figure caption and citation for the preceding image starts]: Midfoot ulcer in a patient with Charcot arthropathy (midfoot collapse)From the collection of Dr Neal R. Barshes; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Midfoot ulcer in a patient with Charcot arthropathy (midfoot collapse)From the collection of Dr Neal R. Barshes; used with permission [Citation ends].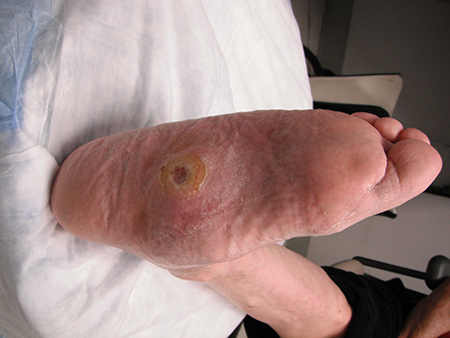 [Figure caption and citation for the preceding image starts]: A foot infection originating from a gangrenous third toe. Note the erythema and fluctuance in the midfoot. An abscess cavity was found tracking under the longitudinal section of macerated skinFrom the collection of Dr Neal R. Barshes; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: A foot infection originating from a gangrenous third toe. Note the erythema and fluctuance in the midfoot. An abscess cavity was found tracking under the longitudinal section of macerated skinFrom the collection of Dr Neal R. Barshes; used with permission [Citation ends].
When examining any infected foot ulcer, it is essential to consider whether there is underlying osteomyelitis, as its presence greatly increases the risk of amputation.[40] Osteomyelitis is particularly likely in wounds that are wide, deep, have been present for many weeks, are located over a bony prominence, show visible bone, or are accompanied by an erythematous, swollen (‘sausage’) toe.[40]
To assess for osteomyelitis, perform a probe-to-bone test as part of the physical examination, by gently inserting a sterile blunt metal probe into the wound. The test is defined as positive if the user feels a hard, gritty structure (i.e., bone) against the end of the probe. Although its reliability varies by users’ technique and experience, overall the probe-to-bone test is the most useful examination technique for diagnosing osteomyelitis in diabetic foot infections (in conjunction with imaging and serum biomarkers), according to the IWGDF and IDSA. Its sensitivity and specificity are 0.87 and 0.83, respectively, suggesting that a positive result in a high-risk patient supports the diagnosis, whereas a negative result in a low-risk patient helps rule it out.[40]
For more information, see Assessing severity of infection and Diagnostic criteria.
Pedal pulse examination
Pulse examination is the most accessible modality for evaluating arterial blood flow to the foot; however, even when performed by an experienced physician such as a vascular surgeon, inter-observer agreement is modest.
The examination can be further impaired by the foot and ankle oedema that is common in a patient with a foot infection.
Nevertheless, the ability to palpate normal pedal pulses usually indicates adequate arterial perfusion to the foot. Absent or weak pulses in the presence of an active ulcer should prompt referral for evaluation and non-invasive testing.
Augmenting the examination with a handheld continuous-wave Doppler probe provides additional information when properly performed and interpreted. This is a non-invasive test.[41]
While monophasic signals do suggest significant peripheral arterial disease, biphasic signals do not exclude it.
In addition to pedal pulse examination, the American Diabetes Association recommends assessing capillary refill time, rubor on dependency, pallor on elevation, and venous filling time as other potential markers of peripheral arterial disease.[37]
Investigations
The diagnosis of foot complications in a person with diabetes is fundamentally a clinical diagnosis based on thorough history and examination: no blood tests are universally recommended, beyond those which form part of routine diabetes care. However in all patients with a suspected foot infection, initial investigations should include a full blood count (to assess for leucocytosis), blood glucose level, and inflammatory biomarkers such as C-reactive protein, erythrocyte sedimentation rate, or procalcitonin.[40] Renal function tests may provide prognostic information; presence of chronic kidney disease increases risk of amputation and all-cause mortality.[42][43] Renal function tests can also be helpful in determining the feasibility of giving iodinated contrast for arterial imaging (if necessary). See Acute kidney injury (Prevention).
Consider x-rays to determine the extent of diabetes-related foot problems if the clinical examination is suggestive of any bone or joint deformities, in particular if suspecting osteomyelitis or acute Charcot's neuro-osteoarthropathy (ideally a weight-bearing view).[9]
Characteristic radiographic features of osteomyelitis include loss of bone cortex, focal loss of trabecular pattern, periosteal reaction, bone sclerosis, and abnormal soft tissue density in the subcutaneous fat suggesting a deep ulcer or sinus tract.[40] Plain x-rays are less sensitive during the acute phase of osteomyelitis, and should be repeated in 2-3 weeks if suspicion is still high after a normal initial x-ray.[40] Although x-ray remains first line for diagnosing osteomyelitis due to its low cost and widespread availability, further imaging may be required, particularly to differentiate from non-infectious structural changes related to Charcot's neuro-osteoarthropathy. Magnetic resonance imaging of the foot is considered the best imaging test for this purpose and is recommended by NICE and the IWGDF when the initial x-ray is normal and the clinical suspicion of osteomyelitis remains.[9][40] 18F-fluorodeoxyglucose positron emission tomography (FDG-PET/CT), 99mTc-exametazime Hexa Methyl Propylene Amine Oxime (HMPAO)-labelled white blood cell scintigraphy, or 99mTc-labeled Ubiquicidin (UBI) SPECT/CT single photon emission computed tomography (SPECT/CT) can be considered as alternatives to MRI for diagnosing osteomyelitis.[40]
If a diabetic foot infection is suspected and a wound is present, NICE recommends sending a soft-tissue or bone sample from the base of the debrided wound for microbiological examination.[9] Tissue specimens collected via curettage or biopsy provide culture results with higher specificity and sensitivity than superficial swabs, although are more burdensome to collect.[40]
If this cannot be obtained, then a deep-tissue swab should be taken to help guide choice of antibiotic.[9]
In low-resource settings, a Gram-stain smear may be used as an alternative to culture to visualise the class of causative pathogen.[40]
Peripheral arterial disease (PAD)
If physical examination of a patient with a diabetic foot ulcer finds anything other than clearly palpable pulses (e.g., weak pulses, examination limited by oedema), order non-invasive vascular testing (ankle/toe pressures or arterial waveforms) for the assessment of PAD.[11]
In the UK, NICE recommends calculating resting ankle-brachial pressure index (ABI) in patients with suspected peripheral arterial disease.[9] Results may be falsely elevated in patients with diabetes because of calcified arteries. Therefore, never rule out a diagnosis of PAD in a patient with diabetes solely based on a normal or raised ABI.[41]
The IWGDF notes that no one test has been found to reliably exclude PAD in patients with a diabetic foot ulcer. Its 2023 guidelines, joint with the European Society for Vascular Surgery and Society for Vascular Surgery, recommend evaluation of pedal Doppler waveforms in combination with ankle systolic pressure, ABI, toe systolic pressure, and toe brachial index (TBI). PAD is less likely if ABI is 0.9 to 1.3, TBI is ≥0.70, and triphasic or biphasic pedal Doppler waveforms are present.[11]
The American Diabetes Association (ADA) recommends performing Doppler ultrasound with pulse volume recordings and ankle/toe pressures in any patient with a history and examination suggestive of PAD. ABIs should be calculated but interpreted carefully, as results can be inaccurate in people with diabetes due to arterial calcification. Toe systolic blood pressure is preferred, as this is more accurate than ABI alone: toe pressures <30 mmHg are suggestive of PAD and poor ulcer healing. Individuals with abnormal pulse volume tracings and toe pressures <30 mmHg with foot ulcers should be referred for immediate vascular evaluation.[37]
Guidelines from the American College of Cardiology Foundation and the American Heart Association state that a resting ABI is indicated in patients who have non-healing foot ulcers, as well as patients with exertional leg symptoms, patients aged 50 years or older with diabetes or a history of smoking, and all other patients aged 65 years and older, in order to establish a diagnosis of lower extremity peripheral arterial disease.[44]
When considering a revascularisation procedure in a patient with PAD and a diabetic foot ulcer, arterial imaging should be performed to provide detailed anatomical information of the lower limb vasculature including the presence, severity, and distribution of arterial stenoses.[11] Arterial imaging should extend all the way from the aorta to the foot, with detailed imaging of the tibial and pedal vessels in particular.
Arterial duplex ultrasound is non-invasive and is recommended by NICE as first-line imaging for all people with PAD for whom revascularisation is being considered.[41] Visualisation is, however, hampered by multi-segment disease and extensive arterial calcification. Angiography is often preferred to duplex ultrasound for this purpose: modalities include computed tomographic angiography, magnetic resonance angiography, and catheter digital subtraction angiography.[11] Of these, catheter digital subtraction angiography is considered the gold standard, according to the joint guidelines from the IWGDF, the European Society for Vascular Surgery and the Society for Vascular Surgery.[11] However, the guidelines note that each technique has its advantages and disadvantages, and the choice depends on local availability and expertise.
For more information, see Peripheral arterial disease.
Classification of diabetic foot ulcers
A diabetic foot ulcer is a break in the skin that includes as a minimum the epidermis and part of the dermis and which occurs below/distal to the malleoli in a person with diabetes.[1] By history and clinical examination, diabetic foot ulcers can be classified as neuropathic, neuro-ischaemic (a combination of neuropathy and ischaemia), or ischaemic.[36] The majority of foot ulcers are purely neuropathic or neuro-ischaemic. Only a small percentage are purely ischaemic: these tend to be painful and follow from minor trauma.[36]
The SINBAD (Site, Ischaemia, Neuropathy, Bacterial Infection, Area, and Depth) scoring system is recommended by the IWGDF for assessing and classifying diabetic foot ulcers, as well as for audit, benchmarking, and communication between healthcare professionals.[7][45] It is well validated and simple to use, and contains the majority of prognostic clinical features that predict outcome including likelihood of amputation. SINBAD is also recommended by NICE and is the system used by the UK National Diabetes Foot Care Audit.[9][46] SINBAD uses a scoring system with a maximum of 6 points. A score of 3 or more is associated with an increased time to healing and greater risk of eventual failure to heal.[45] When using the SINBAD system for communication between healthcare professionals, clinicians should describe the individual variables rather than a total score.[7]
The WIfI (Wound, Ischaemia, Foot Infection) scoring system is also widely used for classifying and describing diabetic ulcers, and is recommended for this purpose by the American Heart Association on the grounds that it also helps determine amputation risk and aids clinical decision-making.[18] The IWGDF also endorses the WIfI system as a valid alternative to SINBAD for classifying and describing diabetic ulcers, provided there is sufficient expertise and resources to use it.[7] As with SINBAD, it is recommended that clinicians report the individual variables that make up the WIfI system, rather than giving a total score, when communicating between healthcare professionals.
To classify the severity of an infected diabetic foot ulcer, the IWGDF recommends use of the IWGDF/InfectiousDiseases Society of America system as the first-choice option to determine whether the infection is mild, moderate, or severe. This system is also recommended by NICE in the UK.[9]
No scoring systems are currently recommended by the IWGDF for predicting the outcome of an ulcer in a specific individual.[7]
For more details on these severity classification systems, see Diagnostic criteria.
Assessing severity of infection
In a person with diabetes and an infected foot ulcer, the IWGDF recommends the IWGDF/IDSA system to classify the severity of infection and guide infection management.[7] This system is also recommended by NICE in the UK.[9]
Infection severity
1: Uninfected
No systemic or local symptoms or signs of infection
2: Mild
IWGDF/IDSA: presence of ≥2 of the following: local swelling or induration, erythema 0.5 to <2.0 cm around the wound, local tenderness or pain, local increased warmth, or purulent discharge (exclude other causes of inflammatory response, such as trauma, gout, acute Charcot's neuro-osteoarthropathy, fracture, thrombosis, and venous stasis).[40]
3: Moderate
IWGDF/IDSA: infection (as for mild severity above) with no systemic manifestations and involving erythema extending ≥2 cm from the wound margin, and/or tissue deeper than skin and subcutaneous tissues (e.g., tendon, muscle, joint, and bone). Add 'O' for any infection involving bone (osteomyelitis).[40]
4: Severe
IWGDF/IDSA: any foot infection with associated manifestations of systemic inflammatory response syndrome, as manifested by ≥2 of the following: temperature >38°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min or PaCO₂ < 4.3 kPa (32 mmHg), WBC count >12 × 10⁹ cells/L (12,000/microlitre) (leukocytosis) or <4 × 10⁹ cells/L (4000/microlitre) (leukopenia); or a normal WBC count with >10% immature (band) forms. Add 'O' for any infection involving bone (osteomyelitis).[40]
Making an accurate diagnosis of osteomyelitis in a diabetic foot can be difficult as there is no universally accepted definition or criteria, and low levels of agreement between commonly used diagnostic tests. Nevertheless, based on current evidence, the IWGDF and IDSA recommend a combination of probe-to-bone test, plain x-ray (and/or further imaging such as MRI), and serum inflammatory markers to support the diagnosis.[40] Bone biopsy is potentially definitive but not widely available, and lacks data demonstrating clear benefits to its use.[40]
Use of this content is subject to our disclaimer