Complications
The desired healing rate is at least a 50% wound area reduction within 4 weeks. The most common reasons for delayed wound healing are subtle infection (especially osteomyelitis), inadequate arterial perfusion, and inadequate offloading.
Clinicians should consider further evaluation for these aetiologies using tests with higher sensitivity (e.g., magnetic resonance imaging or bone biopsy if plain x-ray alone does not suggest osteomyelitis; diagnostic angiogram if non-invasive testing does not suggest arterial insufficiency; non-removable offloading footwear such as a total contact cast or non-removable cast-walker in patients previously provided removable footwear).
Osteomyelitis (infection of the cortical and/or trabecular bone) may occur when chronic ulcers allow for the entry of bacteria into bone. Osteomyelitis is particularly likely in wounds that are wide, deep, have been present for many weeks, are located over a bony prominence, show visible bone, or are accompanied by an erythematous, swollen (‘sausage’) toe.[40]
Most cases are polymicrobial, and involvement of gram-positive and -negative organisms is common.
If osteomyelitis is suspected, the wound may be probed gently with a blunt sterile metal instrument (probe-to-bone test), although this must only be done by skilled clinicians as it can cause harm. The feeling of gritty bone at the base of the ulcer is a positive probe-to-bone test, which supports the diagnosis.
Making an accurate diagnosis of osteomyelitis in a diabetic foot can be challenging as there is no universally accepted definition or criteria, and low levels of agreement between commonly used diagnostic tests. Nevertheless, based on current evidence, the IWGDF and IDSA recommend a combination of probe-to-bone test, plain x-ray (and/or further imaging such as MRI), and serum inflammatory markers to support the diagnosis.[40] Bone biopsy is potentially definitive but not widely available, and lacks data demonstrating clear benefits to its use.[40]
The probe-to-bone test cannot be used in isolation to diagnose or rule out osteomyelitis.[90] The UK National Institute for Health and Care Excellence indicates that osteomyelitis may be present in a person with diabetes despite a negative probe‑to‑bone test.[9]
Management of osteomyelitis may consist of surgical resection of the bone, but oral or parenteral antibiotics alone may be appropriate in selected patients. Up to 6 weeks of antimicrobial therapy is recommended and appears to be as effective as 12 weeks.[9][91] However, a prospective randomised non-inferiority pilot trial found a post-debridement systemic antibiotic therapy course for diabetic foot osteomyelitis of 3 weeks gave similar (and statistically non-inferior) incidences of remission and adverse events to a course of 6 weeks.[92]
Should be identified by the patient (during daily foot exams) or the provider (during clinic follow-up).
Recurrence in the location of a previous ulcer that had completely healed is often due to suboptimal foot biomechanics (i.e., an improper distribution of pressure across the weight-bearing surfaces of the foot).
Management should be similar to that of an initial foot ulcer.
Charcot's neuro-osteoarthropathy (CNO) is a complex disorder that occurs in people with diabetes who have some form of peripheral neuropathy. It is characterised by inflammation and varying degrees of structural damage to the bones, joints, and soft tissue of the foot. The true prevalence is unknown as it is thought to be under-reported; however, estimates range from 0.04% to 0.53% of people with diabetes.[29] The condition can lead to permanent bone and joint deformities, including collapse of the longitudinal and transverse arches of the foot.[93] These deformities predispose to ulceration and infection, which in turn increase the risk of major lower limb amputation.[29]
The diagnosis of CNO should be considered if there is swelling, redness, warmth, or deformity (particularly if the skin is intact). It should be considered even if pain is not present or deformity is not apparent.[9] It may be triggered by trauma, preceding foot surgery, an infected foot ulcer, or offloading the contralateral foot.[94] Pain, erythema, and redness may appear acutely, mimicking a foot infection. Chronic deformation may lead to repetitive trauma of the midfoot (arch) during walking, leading to ulceration in this area.
In the UK, the National Institute for Health and Care Excellence recommends that to confirm the diagnosis the person should be referred within 1 working day to the multidisciplinary foot care service for triage within 1 further working day. Non-weight-bearing should be instituted until definitive treatment can be started. If Charcot's neuro-osteoarthropathy is suspected, a weight-bearing x-ray of the foot and ankle should be performed. If this is normal and CNO is still suspected, magnetic resonance imaging should be performed.[9][95] The mainstay of treatment is offloading with a non-removable offloading device. If a non-removable device is not advisable because of clinical or other circumstances, treatment with a below-knee removable device can be considered.[9] Bisphosphonates should not be offered except as part of a clinical trial.[9]
Monitoring of treatment should be done by clinical assessment, temperature monitoring, and/or repeated x-rays. People who develop subsequent deformity are considered at high risk for future foot ulceration and, once the active CNO arthropathy has become inactive, should have foot orthoses provided, and should be followed-up by a foot protection service.[9]
The International Working Group on the Diabetic Foot (IWGDF) advises using infrared thermometry to calculate the temperature difference between both legs, if suspecting a diagnosis of CNO.[29] Serial skin temperature measurements can also be used to monitor disease activity and help decide when CNO is in remission, in combination with imaging and the presence of soft tissue oedema.
If MRI is unavailable or contraindicated, a nuclear imaging (scintigraphy), computed tomography, or SPECT-CT (single photon emission computed tomography) scan can be used to support the diagnosis of CNO when x-rays are normal but clinical suspicion remains high.[29] Serum biomarkers such as c-reactive protein, erythrocyte sedimentation rate, or white blood cell count, should not be used to diagnose or exclude CNO.[29]
The IWGDF recommends using a non-removable knee-high device to immobilise and offload the foot to promote remission in patients with active CNO and intact skin.[29] Alternative options include a total contact cast, a knee-high walker rendered non-removable, or a removable knee-high device. However below-the-ankle offloading devices, such as surgical or custom-moulded shoes, should not be used as they do not sufficiently offload the diseased bone and joints.[29]
[Figure caption and citation for the preceding image starts]: Midfoot ulcer in a patient with Charcot arthropathy (midfoot collapse)From the collection of Dr Neal R. Barshes; used with permission [Citation ends].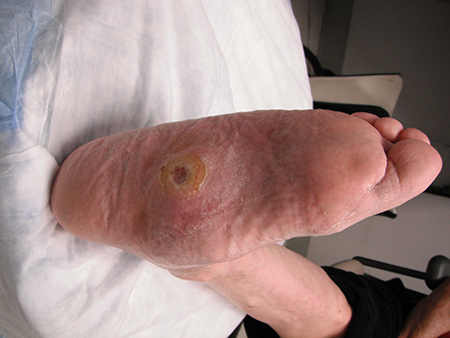
Stenosis requiring endovascular or surgical intervention occurs in about 20% of vein graft bypasses within the first 2 years after creation.[96] Most vascular surgeons will perform routine ultrasound surveillance to identify such stenosis, although the benefits of this approach remain uncertain and local protocols vary.[11]
Although graft thrombosis is often asymptomatic, it may occasionally cause obvious signs of ischaemia, including delayed wound healing, ischaemic rest pains, clinical signs of acute limb ischaemia such as acute-onset weakness, paraesthesias, or limb pain (especially if the vascular reconstruction was done with a prosthetic vascular graft). Incidence may be as high as 10% to 15% during the first year after revascularisation and <5% per year during subsequent years.[96]
Use of this content is subject to our disclaimer