Aetiology
Cerebral arteriovenous malformations (AVMs) are congenital vascular lesions consisting of direct connections between cerebral arteries and veins.
Successful vasculogenesis and angiogenesis in the fetal brain requires complex, perfectly timed interaction of migration, apoptosis, differentiation, and proliferation. These cellular behaviours are coordinated by a variety of growth factors, inflammatory mediators, cell adhesion molecules, extracellular matrix proteins, matrix metalloproteinase enzymes, and hormones. Genes encoding these proteins have altered expression in association with AVMs; it is likely that many gene products have to be incorrectly expressed for an AVM to develop. In particular, vascular endothelial growth factors, the transforming growth factor family, angiopoietins, and particular integrins seem to play a role in AVM development.
While AVMs are congenital, they are dynamic lesions that may grow or regress. Hence, patients often present as young adults rather than children. This is thought to be due to upregulation of angiogenic factors in response to the hypoxic environment surrounding the AVM (ischaemic angiogenic stimulation). Microhaemorrhages within the AVM may also stimulate angiogenesis (haemorrhagic angiogenic proliferation).[26]
Pathophysiology
The response to shunting of arterial blood into veins is the development of 'arterialised' veins with proliferation of smooth muscle and elastin in the vessel wall. Pathological examination of arteriovenous malformations (AVMs) reveals a mass of abnormal vascular channels with widely varying calibre, from hypertrophied to thin-walled sinusoidal vessels with varying degrees of arterialisation. Haemorrhage may be related to the feeding arterial pressure and venous pressure that presumably exceeds the tolerable transmural pressure of the AVM channels.[27]
Cerebral AVMs present a parallel, high-pressure vascular circuit that causes local arterial hypotension and venous hypertension, challenging the local cerebro-vascular physiology and autoregulation. Some authors maintain that local autoregulation is lost as a result of AVMs, whereas others suggest that AVMs develop as a result of loss of autoregulation.[27][28]
When local autoregulation is preserved, the lower limit of the autoregulation curve is displaced to the left in an attempt to maintain normal cerebral blood flow with arterial hypotension (adaptive autoregulatory displacement).[29] The consequent ischaemia/hypoxia in the environment surrounding the AVM may cause neurological deficits, seizure activity, or cognitive impairment. This 'vascular steal phenomenon' is controversial. Brain ischaemia does occur around some, particularly high-flow, low-resistance, AVMs but no definite relationship has been demonstrated between this and focal neurological deficits.[30][31]
Classification
Spetzler-Martin grading system[6]
The Spetzler-Martin grading system is used to predict the risk of surgery and is the most widely used system. Three variables are considered:
Size
Small: <3 cm (1 point)
Medium: 3 to 6 cm (2 points)
Large: >6 cm (3 points).
Pattern of venous drainage
Deep (1 point)
Superficial (0 points).
Neurological eloquence of the brain at the arteriovenous malformation (AVM) location
Eloquent (1 point): areas of the brain that control speech, motor function, and senses; if injured, result in disabling neurological deficits
Non-eloquent (0 points).
The grade is the cumulative total of points allocated for each variable. Poorer surgical outcomes are associated with higher grades.
Grade 1: 4% chance of poor outcome
Grade 2: 10% chance of poor outcome
Grade 3: 18% chance of poor outcome
Grade 4: 31% chance of poor outcome
Grade 5: 37% chance of poor outcome.
The modified Spetzler-Martin grading system subdivides grade 3 AVM according to size: grade 3A (>3 cm) and grade 3B (<3 cm).[7] These subdivisions can be further divided with respect to eloquence and drainage. Applying the modified Spetzler-Martin grading system, grade 3 AVM of small size (1 point) + eloquent location (1 point) + deep drainage (1 point) is most amenable to surgery. The most surgically challenging grade 3 AVM combinations for treatment are medium size (2 points) + non-eloquent location (0 points) + deep drainage (1 point), or medium size (2 points) + eloquent location (1 point) + superficial drainage (0 points). Grade 3 AVMs of large size (3 points), but non-eloquent location and superficial drainage, are not well studied.[8]
The Spetzler-Ponce system simplifies the Spetzler-Martin grading system by consolidating grades as follows:[9]
[Figure caption and citation for the preceding image starts]: Consolidation of the Spetzler-Martin grading system into the Spetzler-Ponce grading systemCreated by the BMJ Knowledge Centre [Citation ends].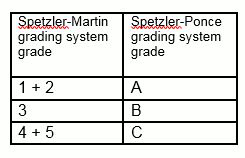
The predictive accuracies for surgical outcomes for the Spetzler-Martin system and the simpler Spetzler-Ponce systems are equivalent, with receiver operating characteristic (ROC) curve areas of 0.711 and 0.713, respectively.
Lawton-Young supplementary grading system[10]
The Lawton-Young scale includes several variables that are not present in the Spetzler-Martin grading system:
Age
<20 years (1 point)
20 to 40 years (2 points)
>40 years (3 points).
Haemorrhagic presentation
No (1 point)
Yes (0 points).
Diffuse nidus
Yes (1 point)
No (0 points).
This scale can be used on its own or as an adjunct to the Spetzler-Martin grading system.[10] While predictive accuracy is greater for the Lawton-Young grading system model than for the Spetzler-Martin grading system (ROC curve area 0.73 vs 0.66, respectively), the full multivariable model (Lawton-Young grading system adjunctive to Spetzler-Martin grading system) has the highest predictive accuracy (ROC curve area 0.78).[10] Unlike with the Spetzler-Martin grading system, where grading takes place before treatment, grading using the Lawton-Young system can change with time and treatment: for example, after radiosurgery the lesion can lose its diffuseness, reducing the overall score by one point.
University of Toronto scoring system[11]
The University of Toronto scoring system uses the relative weights of three variables to predict outcome.
Eloquent location
Yes (4 points)
No (0 points).
Diffuse nidus
Yes (3 points)
No (0 points).
Deep venous drainage
Yes (2 points)
No (0 points).
This grading scale has a predictive ability (ROC curve area 0.79) similar to that of the 10-point supplemented Spetzler-Martin scale (ROC curve area 0.78), and better than that of the original Spetzler-Martin scale (ROC curve area 0.69). This scale discriminates the percentage probability of incurring an early disabling neurological outcome as follows:
0 to 2 points: 1.8%
3 to 5 points: 17.4%
6 to 7 points: 31.6%
>7 points: 52.9%.
HDVL grading scale
HDVL for haemorrhage, diffusion, vein and lesion to eloquence distance. Grading is based on functional magnetic resonance imaging (MRI) and diffusion tensor imaging results.[12]
Variables
Lesion to eloquence distance (LED)
>10 mm: 1 point
5-10 mm: 2 points
<5 mm: 3 points.
Diffuseness
Yes: 1 point
No: 0 points.
Deep draining veins
Yes: 1 point
No: 0 points.
Haemorrhagic history
Yes: 0 points
No: 1 point.
Total score = LED + diffuseness + Deep draining veins + previous haemorrhage
HDVL scores 1-3: operation is recommended
HDVL scores 4-6: individualised multimodal treatment or conservative management is recommended.
Embolisation grading scales
Similar to surgical grading scales, embolisation scales help to predict probability of success and risk of complications.
The arteriovenous malformation embocure scoring (AVMES) system was developed to assess the risk of complications for a curative embolisation of an arteriovenous malformation (AVM) with a non-adhesive liquid embolic agent comprising ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (Onyx).[13]
AVM embocure score:
Size of nidus
<3 cm (1 point)
3 to 6 cm (2 points)
>6 cm (3 points).
Number of arterial feeders
1 to 3 pedicles (1 point)
4 to 6 pedicles (2 points)
>6 pedicles (3 points).
Draining veins
1 to 3 veins (1 point)
4 to 6 veins (2 points)
>6 veins (3 points).
Vascular eloquence, defined as emergence of small and short arterial pedicles from the parent vessel whose occlusion would cause severe complications (i.e., <20 mm from ICA or M1) or too small for microcatheterisation
Present (1 point)
Absent (0 points).
Probabilities
3 points: 100% successful obliteration
4 points: 75% successful obliteration; 8% morbidity
5 points: 78% successful obliteration; 11% morbidity
>5 points: 20% successful obliteration; 30% morbidity.
Stereotactic radiosurgery grading scales
While small AVM size, non-eloquent location, low-flow pattern, and absence of perinidal angiogenesis are predictors of obliteration by stereotactic radiosurgery (SRS),[14] scoring systems exist to calculate the risks of SRS. Two of the most commonly used are the Pollock-Flickinger score and the Virginia radiosurgery scale.[15][16]
Pollock-Flickinger score
0.1 x volume + 0.02 x age + 0.5 x location (hemisphere, corpus callosum, cerebellar = 0 points; basal ganglia, thalamus, brainstem = 1 point)
Probabilities
<0.75: 100% excellent outcomes
>2: 39% excellent outcomes.
Virginia radiosurgery scale
AVM volume
2 to 4 cm³ (1 point)
>4 cm³ (2 points)
History of haemorrhage
Present (1 point)
Absent (0 points).
Eloquent location
Yes (1 point)
No (0 points).
Probabilities
0 or 1 point: 80% good outcomes with SRS
2 points: 70% good outcomes with SRS
3 or 4 points: 45% good outcomes with SRS.
Use of this content is subject to our disclaimer