Approach
Clinical history and presentation can vary significantly depending on the age of the patient and the size of the defect. Patients are usually asymptomatic and are evaluated for an interatrial communication because of a heart murmur, or an abnormal ECG or chest x-ray ordered for other clinical reasons.
History
Depending on the degree of shunting, a child may experience dyspnoea, fatigue, failure to thrive, or recurrent lower respiratory infections.
Occasionally, infants may have symptoms of heart failure. If the interatrial communication occurs in combination with other defects associated with a left-to-right shunt, such as a patent ductus arteriosus or a ventricular septal defect, the defect is often found much earlier due to a higher incidence of heart failure.
Adults may be asymptomatic, but frequently develop symptoms beyond their fourth decade of life including palpitations or dizziness due to atrial arrhythmias, fatigue, dyspnoea, shortness of breath, or exercise intolerance.[23] Pregnancy may precipitate atrial arrhythmias in women with interatrial communications.
Physical exam
The physical findings of an interatrial communication are related to the size of the shunt. Patients may have a hyperdynamic right ventricular impulse by palpation, especially in older children and adults with a large left-to-right shunt. The first heart sound is usually normal, but the second heart sound is often split and does not become single with expiration (fixed split). Patients usually have a systolic ejection murmur best heard at the left upper sternal border and radiating to the back. This murmur is caused by the excess volume of blood crossing the pulmonary valve (functional pulmonary valve stenosis). With moderate or larger defects, an additional mid-diastolic murmur may be appreciated along the lower sternal border due to the volume of blood crossing the tricuspid valve. A murmur of left atrioventricular valve insufficiency is often heard in patients with an ostium primum defect.
When pulmonary hypertension has developed, the volume of the left-to-right shunt decreases and results in loss of fixed splitting of the second heart sound, increased intensity of the pulmonary component of the second heart sound, shortening of the systolic murmur, and disappearance of the diastolic murmur. If the shunt reverses, the patient will appear cyanosed and may develop finger clubbing.
Initial tests
Echocardiography
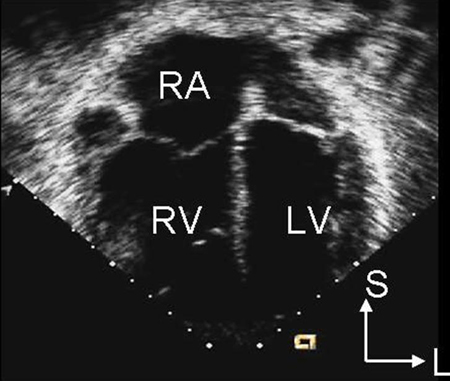
Echocardiography is the preferred imaging modality. Secundum atrial septal defects can often be diagnosed using transthoracic echocardiography, especially in children. Trans-oesophageal echocardiography is used to help assess the size of the defect, to determine the adequacy of tissue rims for device closure in secundum defects, to diagnose sinus venosus-type defects, and to ensure normally connected pulmonary veins. Trans-oesophageal echocardiography is often necessary in older patients if the transthoracic image quality is poor. Two-dimensional echocardiography demonstrates right atrial and ventricular enlargement, as well as the defect itself, especially for secundum defects. [Figure caption and citation for the preceding image starts]: Apical 4-chamber echocardiographic image demonstrating right ventricular enlargement in a patient with an interatrial communication. L: lateral; LV: left ventricle; RA: right atrium; RV: right ventricle; S: superiorImage courtesy of Patrick W. O'Leary, MD [Citation ends].
 Interatrial communications are best seen in subcostal views due to the septum being orthogonal to the ultrasound beam. Secundum defects result in dropout of the mid portion of the atrial septum, and primum defects result in dropout of the inferior portion, in a four-chamber view from the apex. [Figure caption and citation for the preceding image starts]: Apical 4-chamber echocardiographic image of an ostium primum defect (arrows). LA: left atrium; LV: left ventricle; RA: right atriumImage courtesy of Patrick W. O'Leary, MD [Citation ends].
Interatrial communications are best seen in subcostal views due to the septum being orthogonal to the ultrasound beam. Secundum defects result in dropout of the mid portion of the atrial septum, and primum defects result in dropout of the inferior portion, in a four-chamber view from the apex. [Figure caption and citation for the preceding image starts]: Apical 4-chamber echocardiographic image of an ostium primum defect (arrows). LA: left atrium; LV: left ventricle; RA: right atriumImage courtesy of Patrick W. O'Leary, MD [Citation ends]. Superior sinus venosus defects are defined by an interatrial communication in the posterosuperior portion of the atrium, with the superior caval vein at times overriding the defect. Coronary sinus defects have a communication at the orifice of the coronary sinus.
Superior sinus venosus defects are defined by an interatrial communication in the posterosuperior portion of the atrium, with the superior caval vein at times overriding the defect. Coronary sinus defects have a communication at the orifice of the coronary sinus.Doppler examination is used to characterise the shunt volume and to determine the flow pattern through the defect. Non-invasive calculation of the ratio of pulmonary to systemic blood flow, Qp:Qs, can be done with Doppler echocardiography but is no longer used stringently to make clinical decisions. Contrast echocardiography may be used to help determine the presence of a right-to-left shunt (e.g., if a sinus venosus defect cannot be visualised directly).
Chest x-ray
Chest x-ray is not needed to diagnose interatrial communications. It may be normal if there is only a small left-to-right shunt. With larger shunt volumes, the heart may be enlarged and pulmonary vascular markings may be increased. Chest x-ray cannot differentiate between different types of interatrial communications.
ECG
An ECG is not needed to diagnose interatrial communications. It is often normal in secundum, coronary sinus, and sinus venosus-type interatrial communications if the shunt is small. If the shunt is moderate to severe, there may be P waves taller than 2.5 mm, suggesting right atrial enlargement, or R-wave voltages in lead V1 greater than the upper limit of normal for age, suggesting right ventricular hypertrophy. A notch near the apex of the R wave in the inferior limb leads, known as the crochetage pattern, is also found in patients with interatrial communications.
Some ECG findings are specific for particular defects. Primum-type defects frequently produce a counterclockwise frontal-plane loop and left-axis deviation indicating the morphological substrate of a common atrioventricular junction. Sinus venosus defects are associated with a P-wave axis less than 30°.[24]
Subsequent tests
Pulse oximetry
Not diagnostic, but helpful in determining the extent of systemic arterial desaturation because of right-to-left shunting. Desaturation may occur in older patients as a consequence of elevated pulmonary vascular resistance or in patients with left superior vena cava draining directly to the left atrium in unroofed coronary sinus defects.
Chest computed tomography (CT)/magnetic resonance imaging (MRI)
The ability to view structures simultaneously in all 3 dimensions makes CT/MRI valuable in delineating complex congenital heart disease, particularly in patients with limited acoustic windows.[10] Both CT and MR angiography help delineate extracardiac lesions that are not, or inadequately, shown on echocardiography. They may be needed in patients with interatrial communications to help define pulmonary venous anatomy if all pulmonary veins cannot be shown by echocardiography.[10]
Haemodynamic data, direction of intracardiac shunting, and degree of shunting, can be calculated from MRI phase-contrast imaging. In addition, physiological sequelae, including pulmonary artery dilation and pulmonary vascular pruning, can be identified.[10]
Cardiac catheterisation
Cardiac catheterisation is not required for diagnosis. However, it is important in the evaluation of pulmonary vascular resistance in patients with suspected pulmonary vascular obstructive disease; it is considered the gold standard for measuring haemodynamics in patients with congenital heart disease and advanced pulmonary vascular disease, especially to assess operability.[10]
If patients develop a right-to-left shunt, reversibility of the shunt with pulmonary vasodilators should be assessed to guide therapy. If the right-to-left shunt is reversible with pulmonary vasodilators, the interatrial communication can be surgically closed. However, if the right-to-left shunt is irreversible, surgery is not an option.
When available, inhaled nitric oxide, alone or in combination with oxygen, is considered the preferred agent for vasodilator testing. It is safe and short acting and offers a better pulmonary vasodilator effect than systemic agents. However, nitric oxide is not always affordable and thus is not readily available in all cardiac catheterisation laboratories, particularly in low- to middle-income countries. Alternative vasodilator agents include epoprostenol, adenosine, iloprost, treprostinil, milrinone, and glyceryl trinitrate.[10]
Use of this content is subject to our disclaimer